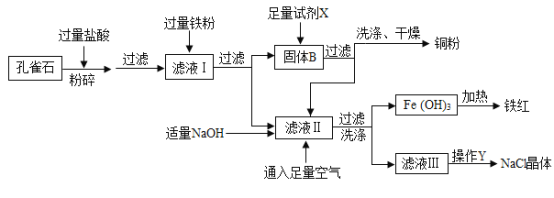
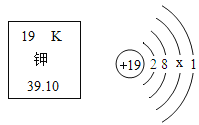
��Ŀ����
����Ŀ��ij�о���ѧϰС���ȡ��NaCl��Na2CO3����25 g���������Ƴ���Һ������������μ���������7.3%��ϡ���ᣬʹ������ȫ�ų������ռ��� 8.8 g CO2���塣
��1������ԭ������Na2CO3����������____________������ϡ�����������____________(д���������)��
��2���±�Ϊ�о���ѧϰС�����������ƵĻ��Һ�з�������μ���(�ӱ���)ϡ���������¼�IJ������ݡ�����������֪Na2CO3��HCl��Ӧ���Է��������У�Na2CO3��HCl=NaCl��NaHCO3��NaHCO3��HCl=NaCl��H2O��CO2����
������ɱ�����δ��IJ��֡�
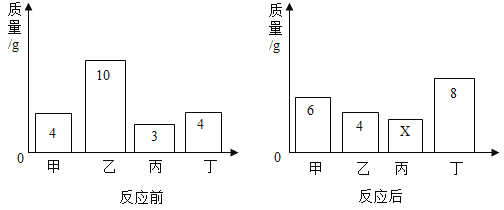
ʵ����� | ÿ�β�����CO2������/g |
��һ������εμ�ϡ����100 g | ________ |
�ڶ�������εμ�ϡ����100 g | 8.8 |
����������εμ�ϡ����100 g | 0 |
�ڸ��ݱ�������������ϵ�л���CO2������(������)��������ϡ���������(������)�Ĺ�ϵͼ________��
���𰸡�84.8% 200 g 0 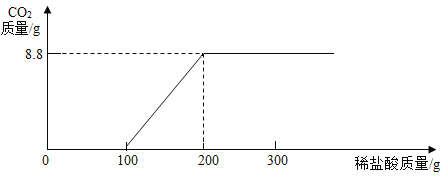
��������
�⣺������̼���Ƶ�����Ϊx������ϡ�����������Ϊy��
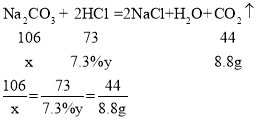
��x=21.2g��y=200g
����ԭ������Na2CO3����������Ϊ��![]() �����ĵ�ϡ�����������Ϊ200g��
�����ĵ�ϡ�����������Ϊ200g��
��2����Ϊ�����������ᣬ�ܹ������Ķ�����̼������Ϊ8.8g�����ݱ��еڶ��μ����100g��������Ķ�����̼Ϊ100g���ʿ�֪�ڵ�һ�μ���ʱ��̼���ƺ�100gϡ����ǡ����ȫ��Ӧ�����Ȼ��ƺ�̼�����ƣ�����δ����������̼��������̼����Ϊ0g�������μ���������ٲ���������̼����Ӧ��ȫ������������ļ�������ӣ�����8.8g���䡣�ʱ�������0��ͼ��Ϊ�� ��
��

 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�����Ŀ��Сӱͬѧ������Ͷ������ͭ��Һ��ʱ���������ɺ�ɫ�����ͬʱ�н϶�����ݷų���Сӱ�Բ������ݵ�����������ʣ�������ȤС���ͬѧ��չ��������̽��ʵ�顣
��������⣩������������ʲô�أ�
���������룩��ȤС���ͬѧ�ֱ����������²��룺С���IJ��룺O2��С��IJ��룺SO2��Сӱ�IJ��룺H2��Сΰ�IJ��룺N2��
���������ۣ�ͬѧ�Ǿ�������һ����ΪСΰ�IJ��벻��ȷ��������_____��
���������ϣ�SO2���д̼�����ζ��������ˮ������NaOH��Һ������Ӧ����Na2SO3��ˮ��
��ʵ��̽����ͬѧ���ռ����壬���ֱ�Ը��ԵIJ��������֤��
ʵ����� | ʵ������ | ʵ����� | |
С�� | �������ǵ�ľ�����뵽�ռ��������� | _____ | ���벻��ȷ |
С�� | ������������ͨ��ʢ��NaOH��Һ��ϴ��ƿ�У��������� | ͨ��ǰ��ϴ��ƿ���������� | ����_____��������ȷ����������ȷ���� |
Сӱ | �������������ü��첣���ܵ������ȼ���������������ձ����ڻ�����Ϸ� | ����ȼ�գ���������ɫ���棬�ձ��ڱ�_____ | ������ȷ |
д��Сӱʵ���з�����Ӧ�Ļ�ѧ����ʽ_____��
��������Ľ���
��1��С����ΪС����Сӱ��ʵ�鷽�������ڰ�ȫ������������_____��
��2��ʵ���������ʦ��Ϊ����Ҫ����ʵ�鼴��ͨ�������ķ�����֤С��IJ��룬����Ϊ��ѡ���Ϊ��㷽����������_____��
����չ�����죩
��1����ʵ��̽���Ʋ⣬��֪����ͭ��Һ�п��ܺ��г����Ե����ʣ�һ�㲻����ɫʯ����Һ��������ͭ��Һ����Ե�ԭ����_____��
��2������ʦ���ܣ�CuSO4�����������ܼӿ��������ķֽ����ʡ�Ϊ����֤��ʦ��˵������Ҫ���е�̽�������У����Ƿ��ܼӿ��������ķֽ⣻��_____��