��Ŀ����
��Լ��Դ�ͱ��������Ѿ���Ϊ���ǵĻ������ߣ����ܼ��š�������������̬�н�������������Щ��Ĺ����ص㣮���᳧��������������ǣ��Ѻ������ȼ�գ����ɶ�������������������ڸ��ºʹ���������������������������������ˮ�����������ᣮд�����������ˮ������������Ļ�ѧ����ʽ______ij������Ʒ�к������������ƣ������ⶨ��̼���Ƶ��������������ú�����������ij����ˮ��������ʵ�飺
[ʵ��ԭ��]Na2CO3+H2SO4=Na2SO4+H2O+CO2��
ͨ��ʵ��ⶨ��Ӧ�����Ķ�����̼���������������ԭ��Ʒ��̼���Ƶ��������������̼��������Ʒ�е�����������
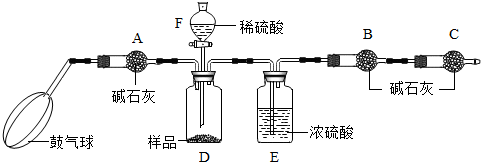
[ʵ��װ��]
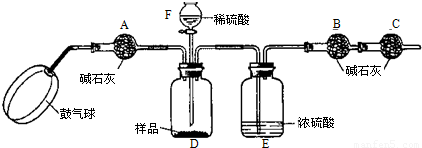
[ʵ�鲽��]
����ͼ����װ�ã���B��C�⣩����������ҩƷ��
�ڳ�������¼B��������m1����������ʱע����B�����ˣ���
�۰������������������Լ1���ӣ�
��������B��C��
�ݴ�Һ©��F�Ļ�������ϡ������ټ���D�кرջ�����
�ް�����������Լ1���ӣ�
�߳�������¼B��������m2����������ʱע����B�����˼�E�Ҷ˵ij��ڣ���
����㣮
��1����֪��ʯ�ҵ���Ҫ�ɷ����������ƺ��������ƣ�������A�������ǣ�______������ʹ�ⶨ���ƫ��
��2��______���ܻ��ܣ���ϡ�������ϡ���ᣬ��Ϊ�������______�ԣ���ʹ���̼���Ƶ���������______����ƫ��ƫС�䣬��ͬ������ȥ�������C������̼���Ƶ�������������______
��3��Eװ�õ�������______��
��4����ʵ���ܷ�ʡ�Ԣۡ����������裿______�����ܻ��ܣ���ԭ��ֱ���______��______��
��5������ȡ��Ʒ������Ϊ6g����Һ©��F��ʢ��5%ֻ������һ�����ʵij����ˮ���Ƶ�m1Ϊ51.20g��m2Ϊ53.40g����������������λС����
��
��1����Ʒ��̼���Ƶ���������Ϊ���٣�
��2��Dװ�������÷�Ӧ��������Һ���������������Ƕ��٣�
���𰸡���������1�����ø����Aͨ�������ų�װ��D�еĿ������ܷ�ֹ������ˮ�����Ͷ�����̼�ĸ��Ž��
��2�����������ܻ�ӷ����Ȼ������壬�Ȼ����ܺͼ�ʯ�ҷ�Ӧ�������C�ܷ�ֹ�����еĶ�����̼��ˮ��������B���
��3������Ũ���������ˮ��������ý��
��4������ǰһ�ι�����Ϊ���ų�D�еĿ�����ֹ�����ж�����̼����ĸ��ţ���һ�ι�����Ϊ�˽����ɵĶ�����̼ȫ���ų����
��5���ٸ��ݷ�Ӧǰ��Bװ�����ص�������ϡ�����̼���Ʒ�Ӧ���ɵĶ�����̼�����������̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ���6g��Ʒ��̼���Ƶ����������ɽ�𣮢ڸ��ݷ�Ӧǰ��Bװ�����ص�������ϡ�����̼���Ʒ�Ӧ���ɵĶ�����̼�����������̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ�����Ӧ���ɵ������ƺ����������ʵ�������Ȼ�����ϡ�����������������Ĺ�ʽ���ϡ������Һ�����������������غ㶨�����������������Һ�����������������Ƶ�����������������Һ����������100%���ɽ��
����⣺��1�������Aͨ�������ų�װ��D�еĿ������ܷ�ֹ������ˮ�����Ͷ�����̼�ĸ��ţ��ʴ𰸣���ֹ������ˮ�����Ͷ�����̼�ĸ��ţ�
��2�������ܻ�ӷ����Ȼ������壬�Ȼ����ܺͼ�ʯ�ҷ�Ӧ�������C�ܷ�ֹ�����еĶ�����̼��ˮ��������B���ʴ𰸣����ܣ��ӷ��ԣ�ƫ��ƫ��
��3��Ũ���������ˮ��������ã��ʴ𰸣���ȥ���ɵĶ�����̼�е�ˮ������
��4��ǰһ�ι�����Ϊ���ų�D�еĿ�����ֹ�����ж�����̼����ĸ��ţ���һ�ι�����Ϊ�˽����ɵĶ�����̼ȫ���ų����ʴ𰸣����ܣ�������Ϊ���ų�D�еĿ�����������Ϊ�˽�D�����ɵĶ�����̼ȫ���ų���
��5����ϡ���������������Ϊx�����ɵ������Ƶ�����Ϊy����Ʒ��̼���Ƶ�����Ϊz��Bװ�����ص���������̼���ƺ�ϡ���ᷴӦ���ɵĶ�����̼������Ϊ��53.40g-51.20g=2.2g
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
106 98 142 44
z x y 2.2g
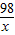 =
=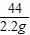
x=4.90g
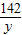 =
=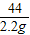
y=7.10g
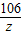 =
=
z=5.3g
��1����Ʒ��̼���Ƶ���������Ϊ��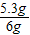 ×100%=88.3%
×100%=88.3%
��2������ϡ���������Ϊ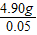 =98g
=98g
������������Һ������Ϊ98g+5.3g-2.2g=101.1g
������������Һ��������������Ϊ��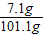 ×100%=7.02%
×100%=7.02%
����Ʒ��̼���Ƶ���������Ϊ88.3%��������Һ�������Ƶ���������Ϊ7.02%
����������ͨ��ʵ��̽�����̿����˼�ʯ�Ҽ�����ˮ���ܺͶ�����̼��Ӧ��Ũ���������ˮ�ԡ�̼���ƺ�ϡ���ᷴӦ���������ơ�ˮ�Ͷ�����̼�����ҿ����˸���̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ�Ľ����Һ�������������ļ��㣬�ѶȽϴ�ֻҪ�������ÿ���IJ���Ŀ�IJ��ܽ����⣮
��2�����������ܻ�ӷ����Ȼ������壬�Ȼ����ܺͼ�ʯ�ҷ�Ӧ�������C�ܷ�ֹ�����еĶ�����̼��ˮ��������B���
��3������Ũ���������ˮ��������ý��
��4������ǰһ�ι�����Ϊ���ų�D�еĿ�����ֹ�����ж�����̼����ĸ��ţ���һ�ι�����Ϊ�˽����ɵĶ�����̼ȫ���ų����
��5���ٸ��ݷ�Ӧǰ��Bװ�����ص�������ϡ�����̼���Ʒ�Ӧ���ɵĶ�����̼�����������̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ���6g��Ʒ��̼���Ƶ����������ɽ�𣮢ڸ��ݷ�Ӧǰ��Bװ�����ص�������ϡ�����̼���Ʒ�Ӧ���ɵĶ�����̼�����������̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ�����Ӧ���ɵ������ƺ����������ʵ�������Ȼ�����ϡ�����������������Ĺ�ʽ���ϡ������Һ�����������������غ㶨�����������������Һ�����������������Ƶ�����������������Һ����������100%���ɽ��
����⣺��1�������Aͨ�������ų�װ��D�еĿ������ܷ�ֹ������ˮ�����Ͷ�����̼�ĸ��ţ��ʴ𰸣���ֹ������ˮ�����Ͷ�����̼�ĸ��ţ�
��2�������ܻ�ӷ����Ȼ������壬�Ȼ����ܺͼ�ʯ�ҷ�Ӧ�������C�ܷ�ֹ�����еĶ�����̼��ˮ��������B���ʴ𰸣����ܣ��ӷ��ԣ�ƫ��ƫ��
��3��Ũ���������ˮ��������ã��ʴ𰸣���ȥ���ɵĶ�����̼�е�ˮ������
��4��ǰһ�ι�����Ϊ���ų�D�еĿ�����ֹ�����ж�����̼����ĸ��ţ���һ�ι�����Ϊ�˽����ɵĶ�����̼ȫ���ų����ʴ𰸣����ܣ�������Ϊ���ų�D�еĿ�����������Ϊ�˽�D�����ɵĶ�����̼ȫ���ų���
��5����ϡ���������������Ϊx�����ɵ������Ƶ�����Ϊy����Ʒ��̼���Ƶ�����Ϊz��Bװ�����ص���������̼���ƺ�ϡ���ᷴӦ���ɵĶ�����̼������Ϊ��53.40g-51.20g=2.2g
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
106 98 142 44
z x y 2.2g
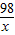 =
=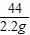
x=4.90g
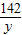 =
=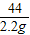
y=7.10g
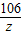 =
=
z=5.3g
��1����Ʒ��̼���Ƶ���������Ϊ��
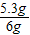 ×100%=88.3%
×100%=88.3%��2������ϡ���������Ϊ
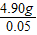 =98g
=98g������������Һ������Ϊ98g+5.3g-2.2g=101.1g
������������Һ��������������Ϊ��
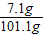 ×100%=7.02%
×100%=7.02%����Ʒ��̼���Ƶ���������Ϊ88.3%��������Һ�������Ƶ���������Ϊ7.02%
����������ͨ��ʵ��̽�����̿����˼�ʯ�Ҽ�����ˮ���ܺͶ�����̼��Ӧ��Ũ���������ˮ�ԡ�̼���ƺ�ϡ���ᷴӦ���������ơ�ˮ�Ͷ�����̼�����ҿ����˸���̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ�Ľ����Һ�������������ļ��㣬�ѶȽϴ�ֻҪ�������ÿ���IJ���Ŀ�IJ��ܽ����⣮

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ