��Ŀ����
����Ŀ��2009��6��1�գ����ҡ�ʳƷ��ȫ������ʽʵʩ����ʳƷ��ҵ���������һ���ѳ�Ϊ��ʷ������ʳƷ�ؼ졢���ϸ���������Ϊ�����ԭ��ij���վ��ijƷ���̷۽��м�⡣ȡ���̷���Ʒ100g��ʹ���е������еĵ�Ԫ����ȫת��ɰ�������50g������������Ϊ9.8%��ϡ����ǡ����ȫ���գ�2NH3+H2SO4����NH4��2SO4�������㲢�ش��������⣺
��1����������������____________��
��2�����̷��к���Ԫ�ص���������__________________��
��3���̷��е����ʺ����Ĺ��ұ�Ϊ��ÿ100g�̷��к�������12g��25g�� �������е�Ԫ�ص�ƽ����������Ϊ16%����ϸ��̷��е�Ԫ�ص�����������ΧΪ______��ͨ���Ա�˵�����������̷��Ƿ�ϸ�____________��
��4�������̷������ϸ�������Ϊʹ�����ϸ����������̷������ӻ���ԭ�������谷����ѧʽC3N6H6�����������̷ۼ���еĺ���������ɵ����ʴ��ļ����������100g�������ϸ��̷���������Ҫ������ٿ������谷�����ܳ�Ϊ���ϸ����̷�__________________������������:2/3-0.0192��0.65��
���𰸡�1.7g 1.4% 1.92%��4% �̷۲��ϸ� 0.8g
��������
��1�����������������Ϊx
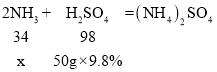
![]()
x =1.7g
��������������Ϊ1.7g
��2�����̷��к���Ԫ�ص��������� =
��3��ÿ100g�̷��к�������12g��25g�� �������е�Ԫ�ص�ƽ����������Ϊ16%����ϸ��̷��е�Ԫ�ص�����������ΧΪ ![]() = 1.92%��4% ��1.4%��1.92% ���̷۲��ϸ�
= 1.92%��4% ��1.4%��1.92% ���̷۲��ϸ�
��4����������Ҫ���������谷������Ϊy
![]()
y =0.8g
��������Ҫ����0.8g�����谷

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�