��Ŀ����
��2013?������һģ����ˮ�̲��ŷḻ����Դ��
��1����ˮ���ܼ� ��
��2���Ӻ�ˮ����ȡ����һ��ɲ���
��3����ͼ1�����������ʹ�õ�Ӧ����ˮ�������øþ�ˮ����2000g���Ȼ���3%�ĺ�ˮ��ɹ4Сʱ���ռ���500g������ˮ����ʱʣ�ຣˮ���Ȼ��Ƶ���������Ϊ
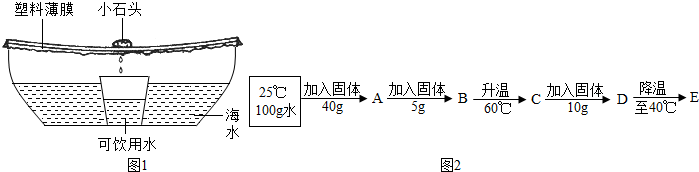
��4�������Ȼ����Ʊ�̼���Ƶ�ͬʱ���Եõ��Ȼ�泥��±�Ϊ�Ȼ�淋��ܽ�ȣ�
����100gˮ�в��ϼ����Ȼ�粒����ı��¶ȣ��õ�ͼ2��Ӧ����ҺA��E��
��ҺD��
�ڽ�ʢ����ҺE��С�ձ�����ʢ��ˮ�Ĵ��ձ��У�����ձ��ڼ���NaOH���壬С�ձ��ڵĹ����ܽ⣬ԭ����
��1����ˮ���ܼ� ��
ˮ
ˮ
����2���Ӻ�ˮ����ȡ����һ��ɲ���
�����ܼ�
�����ܼ�
��������3����ͼ1�����������ʹ�õ�Ӧ����ˮ�������øþ�ˮ����2000g���Ȼ���3%�ĺ�ˮ��ɹ4Сʱ���ռ���500g������ˮ����ʱʣ�ຣˮ���Ȼ��Ƶ���������Ϊ
4%
4%
��
��4�������Ȼ����Ʊ�̼���Ƶ�ͬʱ���Եõ��Ȼ�泥��±�Ϊ�Ȼ�淋��ܽ�ȣ�
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| �ܽ��/g | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 | 65.6 |
��ҺD��
������
������
������͡������͡�����Һ����ҺE����������9.2
9.2
g���ڽ�ʢ����ҺE��С�ձ�����ʢ��ˮ�Ĵ��ձ��У�����ձ��ڼ���NaOH���壬С�ձ��ڵĹ����ܽ⣬ԭ����
�������ƹ�������ˮ���ȣ�ʹ���Ȼ�淋��ܽ��������ܽ�
�������ƹ�������ˮ���ȣ�ʹ���Ȼ�淋��ܽ��������ܽ�
����������1���������ʡ��ܼ��Ķ����жϣ�
��2�������Ȼ����ܽ�����¶ȵı仯���Ʒ����ᾧ�ķ�����
��3��������������������㣻
��4���ٸ����Ȼ�粒�����60�桢40���ǵ��ܽ�ȷ������㣻
�ڸ���������������ˮ�ܷų��ȣ��Ȼ�淋��ܽ�����¶ȵ����߶����������
��2�������Ȼ����ܽ�����¶ȵı仯���Ʒ����ᾧ�ķ�����
��3��������������������㣻
��4���ٸ����Ȼ�粒�����60�桢40���ǵ��ܽ�ȷ������㣻
�ڸ���������������ˮ�ܷų��ȣ��Ȼ�淋��ܽ�����¶ȵ����߶����������
����⣺��1����ˮ���ܼ���ˮ��
��2�������Ȼ��Ƶ��ܽ�����¶ȵ�Ӱ��仯�������ԣ��Ӻ�ˮ����ȡ����һ��ɲ��������ܼ�������
��3��ʣ�ຣˮ���Ȼ��Ƶ���������Ϊ��
��100%=4%
��4�������Ȼ�淋��ܽ�ȱ���֪����60��ʱ�Ȼ�淋��ܽ����55.2g����ͼ2�Ĺ��̿�֪��D��Һ����100g�����ܽ����Ȼ�淋������ǣ�40g+5g+10g=55g��55.2g�����ԣ�D�Dz�������Һ��������40��ʱ�Ȼ�淋��ܽ����45.8g�����ԣ�D���µ�40��ʱ���������������Ϊ��55g-45.8g=9.2g��
�ڽ�ʢ����ҺE��С�ձ�����ʢ��ˮ�Ĵ��ձ��У�����ձ��ڼ���NaOH���壬С�ձ��ڵĹ����ܽ⣬ԭ���ǣ��������ƹ�������ˮ���ȣ�ʹ���Ȼ�淋��ܽ��������ܽ⣮
�ʴ�Ϊ����1��ˮ����2�������ܼ�����3��4%����4���ٲ����ͣ�9.2�����������ƹ�������ˮ���ȣ�ʹ���Ȼ�淋��ܽ��������ܽ⣮
��2�������Ȼ��Ƶ��ܽ�����¶ȵ�Ӱ��仯�������ԣ��Ӻ�ˮ����ȡ����һ��ɲ��������ܼ�������
��3��ʣ�ຣˮ���Ȼ��Ƶ���������Ϊ��
| 2000g��3% |
| 2000g-500g |
��4�������Ȼ�淋��ܽ�ȱ���֪����60��ʱ�Ȼ�淋��ܽ����55.2g����ͼ2�Ĺ��̿�֪��D��Һ����100g�����ܽ����Ȼ�淋������ǣ�40g+5g+10g=55g��55.2g�����ԣ�D�Dz�������Һ��������40��ʱ�Ȼ�淋��ܽ����45.8g�����ԣ�D���µ�40��ʱ���������������Ϊ��55g-45.8g=9.2g��
�ڽ�ʢ����ҺE��С�ձ�����ʢ��ˮ�Ĵ��ձ��У�����ձ��ڼ���NaOH���壬С�ձ��ڵĹ����ܽ⣬ԭ���ǣ��������ƹ�������ˮ���ȣ�ʹ���Ȼ�淋��ܽ��������ܽ⣮
�ʴ�Ϊ����1��ˮ����2�������ܼ�����3��4%����4���ٲ����ͣ�9.2�����������ƹ�������ˮ���ȣ�ʹ���Ȼ�淋��ܽ��������ܽ⣮
��������嫵ĺ�������������ˮ��������̲��ŷḻ�Ļ�ѧ��Դ�������й���Һ��֪ʶ����������������йغ�ˮ����ȡ���ʵ����⣮

��ϰ��ϵ�д�
�����Ŀ
 ��2013?������һģ��ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮
��2013?������һģ��ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮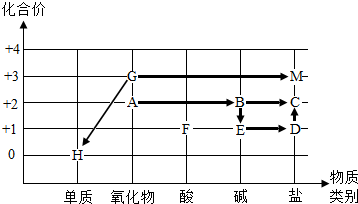 ��2013?������һģ��ͬѧ��������������������ij��Ԫ�صĻ��ϼ۹����˳��л�ѧ�������ʼ��ת����ϵ����ͼ��ͼ�С�������ʾ���ʼ��ת������
��2013?������һģ��ͬѧ��������������������ij��Ԫ�صĻ��ϼ۹����˳��л�ѧ�������ʼ��ת����ϵ����ͼ��ͼ�С�������ʾ���ʼ��ת������ ��2013?������һģ��ʵ�����ù�����ϡ����ʹ���ʯ��ȡCO2��ȡ50g��Ӧ�����Һ����ε���̼������Һ����õ���̼������Һ�����������������������ϵ��ͼ��ʾ��
��2013?������һģ��ʵ�����ù�����ϡ����ʹ���ʯ��ȡCO2��ȡ50g��Ӧ�����Һ����ε���̼������Һ����õ���̼������Һ�����������������������ϵ��ͼ��ʾ��