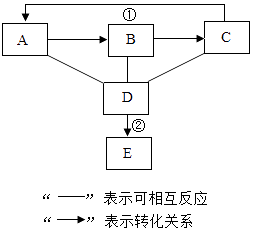
ЬтФПФкШн
ЁОЬтФПЁПЮЊСЫВтЖЈФГЭаПЛьКЯЮяжааПЕФжЪСПЗжЪ§ЃЌФГЭЌбЇРћгУИУКЯН№гыЯЁСђЫсЗДгІЃЌНјааСЫШ§ДЮЪЕбщЃЌЫљЕУЯрЙиЕФЪЕбщЪ§ОнМЧТМШчЯТЃК
ЕквЛДЮ | ЕкЖўДЮ | ЕкШ§ДЮ | |
ЫљШЁКЯН№ЕФжЪСП/g | 25 | 25 | 50 |
ЫљгУЯЁСђЫсЕФжЪСП/g | 120 | 160 | 100 |
ЩњГЩЧтЦјЕФжЪСП/g | 0.4 | 0.4 | 0.4 |
ЂйУПвЛДЮЗДгІжаЩњГЩЧтЦјЕФЮяжЪЕФСПЪЧ ЃЎ
ЂкЪдМЦЫуИУЭаПКЯН№жааПЕФжЪСПЗжЪ§ЃЈаДГіМЦЫуЙ§ГЬЃЉЃЎ
ЂлДгЩЯБэЪ§ОнЗжЮіЃЌЕБЫљШЁКЯН№гыЫљгУЯЁСђЫсЕФжЪСПБШЮЊЪБЃЌБэУїКЯН№жаЕФаПгыЯЁСђЫсжаЕФСђЫсЧЁКУЭъШЋЗДгІЃЎ
ЁОД№АИЁП0.2molЃЛ52%ЃЛ1ЃК4
ЁОНтЮіЁПНтЃКЃЈ1ЃЉУПвЛДЮЗДгІжаЩњГЩЧтЦјЕФЮяжЪЕФСПЪЧЃК ![]() =0.2molЃЛЃЈ2ЃЉЩшZnЕФЮяжЪЕФжЪСПЮЊx
=0.2molЃЛЃЈ2ЃЉЩшZnЕФЮяжЪЕФжЪСПЮЊx
Zn+H2SO4ЁњZnSO4+ | H2Ёќ |
1 | 1 |
x | 0.2mol |
![]() =
= ![]()
x=0.2mol
ЫљвдZn%= ![]() =52%ЃЛЃЈ3ЃЉИљОнБэИёЬсЙЉЕФЪ§ОнПЩвдПДГіЃЌЩњГЩ0.4gЧтЦјЃЌашвЊКЯН№ЕФжЪСПЪЧ25gЃЌашвЊСђЫсЕФжЪСПЪЧ100gЃЌМДКЯН№гыЫљгУЯЁСђЫсЕФжЪСПжЎБШЮЊ25gЃК100g=1ЃК4ЪБЃЌЖўепЧЁКУЭъШЋЗДгІЃЎ
=52%ЃЛЃЈ3ЃЉИљОнБэИёЬсЙЉЕФЪ§ОнПЩвдПДГіЃЌЩњГЩ0.4gЧтЦјЃЌашвЊКЯН№ЕФжЪСПЪЧ25gЃЌашвЊСђЫсЕФжЪСПЪЧ100gЃЌМДКЯН№гыЫљгУЯЁСђЫсЕФжЪСПжЎБШЮЊ25gЃК100g=1ЃК4ЪБЃЌЖўепЧЁКУЭъШЋЗДгІЃЎ
ЙЪД№АИЮЊЃКЃЈ1ЃЉ0.2molЃЛЃЈ2ЃЉ52%ЃЛЃЈ3ЃЉ1ЃК4ЃЎ
ЃЈ1ЃЉИљОнУПвЛДЮЗДгІЩњГЩЕФЧтЦјжЪСПКЭЧтЦјЕФФІЖћжЪСПНјааМЦЫуЃЛЃЈ2ЃЉИљОнаПКЭЯЁСђЫсЗДгІЩњГЩЧтЦјЃЌвРОнЬтжаЕФЧтЦјНјааМЦЫуЃЛЃЈ3ЃЉИљОнБэИёЬсЙЉЕФЪ§ОнЗжЮіЫљвдН№ЪєКЭСђЫсЕФжЪСПЃЌШЛКѓвРОнЗДгІЕФЛЏбЇЗНГЬЪНМЦЫуМДПЩЃЎ

ЁОЬтФПЁПNaOHЪЧЛЏбЇЪЕбщжаГЃгУЕФЪдМСЃЎ
ЃЈ1ЃЉЧтбѕЛЏФЦШмвКгыжИЪОМСЕФЗДгІЃЌЭъГЩШчБэЃЎ
зЯЩЋЪЏШяШмвК | ЮоЩЋЗгЬЊ | |
ЧтбѕЛЏФЦШмвК |
ЃЈ2ЃЉжаКЭЗДгІНЋФГNaOHШмвКж№ЕЮЕЮШывЛЖЈСПЕФФГбЮЫсжаЃЌШчЭМЭМЯѓКЯРэЕФЪЧЃЈЬюЁАМзЁБЛђЁАввЁБЃЉЃЎ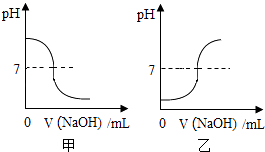
ЃЈ3ЃЉЯђГЄЦкГЈПкЗХжУЕФNaOHШмвКжаЕЮМгбЮЫсЪБЃЌвтЭтЗЂЯжгаЦјХнВњЩњЃЌЧыНтЪЭЦфдвђЃЎ
ЃЈ4ЃЉдѕбљЧјЗжЧтбѕЛЏФЦШмвКгыБЅКЭЪЏЛвЫЎЃЌМђвЊаДГіЪЕбщВНжшЁЂЯжЯѓКЭНсТлЃЎ
ЃЈ5ЃЉГЃгУЪьЪЏЛвгыДПМюжЦШЁЧтбѕЛЏФЦЃЌШєвЊжЦШЁЧтбѕЛЏФЦ80gЃЌМЦЫуашвЊДПМюЖрЩйЃП