��Ŀ����
����Ŀ����7�֣���ҵ�ϲ������ӽ���Ĥ���۵�ⱥ��ʳ��ˮ�����Եõ���Ũ�ȵ��ռ���Һ����NaOH35%��48%����ijѧϰС��Ϊ����֤�����ȼ����������NaOH��Һ�Ƿ�ﵽ��Ũ�ȱ������������²��������������㣺
��1���������ɼ��˵����е�NaOH��Һ100g��NaOH����Ԫ�ص��������� �� ��
��2����ʵ��������ͼ��ʾŨ��������200g24��5%��ϡ���ᣬ������ȡŨ����������������ȡ��������
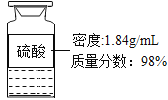
��3�������кͲⶨ����ɼ�������Һ����μ��������Ƶ�ϡ���ᣬ�����ϲⶨ��Һ��pHֵ����pH=7ʱ������ϡ����160g��ͨ�������жϴ�ʱ������NaOH��Һ�Ƿ�ﵽ��Ũ�ȱ���
���𰸡���1��40%��2����ҪŨ��������Ϊ200g��24��5%��98%��1��84g/ml��27ml
(3��μӷ�Ӧ���������Ƶ���������ΪX
H2SO4 + 2NaOH==Na2SO4+2H2O
98 80
160g��24��5% 100gX
98��80=160g��24��5%��100gX
X=32%
32%<35% ���Ը���Һû�дﵽ��Ũ�ȱ�
��������
�����������1�������������ƵĻ�ѧʽ���ɼ�������������Ԫ�ص���������Ϊ�� ![]() ��100%=40%
��100%=40%
��2��ϡ��Ũ����ʱ�����е����ʵ��������䡣����������Ϊ��
��ҪŨ��������Ϊ200g��24��5%��98%��1��84g/ml��27ml
(3)���������֪����ӦΪ�������������Ʒ�Ӧ���������ƺ�ˮ����֪��Ϊ�����������δ֪��Ϊ����������Һ��������������������˼·���ɸ��ݻ�ѧ����ʽ�����������������������ϵ���������Ӧ���������Ƶ�������Ȼ���ٸ�������������������ķ������������������Һ���������������������жϾ������������£�
�⣺��1������������������Ԫ�ص���������Ϊ�� ![]() ��100%=40%
��100%=40%
��2����ҪŨ��������Ϊ200g��24��5%��98%��1��84g/ml��27ml
(3��μӷ�Ӧ���������Ƶ���������ΪX
H2SO4 + 2NaOH==Na2SO4+2H2O
98 80
160g��24��5% 100gX
98��80=160g��24��5%��100gX
X=32%
32%<35% ���Ը���Һû�дﵽ��Ũ�ȱ�
�𣺣�1��������������Ԫ�ص���������Ϊ40%��
��2����ҪŨ��������Ϊ27ml
��3��������NaOH��Һû�дﵽ��Ũ�ȱ���