��Ŀ����
����Ŀ��ijУ����ѧ��ȥ����ɽ���Σ������˼���ʯ��ʯ��Ʒ��Ϊ�˼����Ʒ��̼��Ƶĺ������ס��ҡ���������λͬѧ������������ͬ����������Ʒ��ַ�Ӧ�����вⶨ![]() ������Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ
������Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ![]() ��������������
��������������
�� | �� | �� | �� | |
������ĥ���ʯ��ʯ��Ʒ���� |
|
|
|
|
��������� |
|
|
|
|
ʣ���������� |
|
|
|
|
���ʴ�
��1��![]() ��Ʒ��
��Ʒ��![]() �����ַ�Ӧ�������Ƿ���ʣ��______
�����ַ�Ӧ�������Ƿ���ʣ��______ ![]() ����������������
����������������![]() ����Ʒ��̼��Ƶ�����������______��
����Ʒ��̼��Ƶ�����������______��
��2�����������������������![]() ��������
��������![]()
��3������![]() ��Ʒ��
��Ʒ��![]() �����Ϻ����������̼������������Ĺ�ϵͼ��
�����Ϻ����������̼������������Ĺ�ϵͼ��

���𰸡���1����90%
��2��14.6%
��3��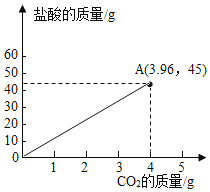
��������
��1���ɱ��е����ݿ�֪��ÿ10g��ϡ�����ܷ�Ӧ��̼��Ƶ�����Ϊ2g�����ʵ�����Ϊ1g����9g̼�����ȫ��Ӧ��Ҫ45.0g���ᣬ��10.0g��Ʒ��45.0g�����ַ�Ӧ������û��ʣ�ࣻ
�ɶ������ݿ�֪�����ʵ�����Ϊ1.0g��10.0g��Ʒ��̼��Ƶ�����Ϊ��![]() ��̼������������ǣ�
��̼������������ǣ�![]() ��
��
��2����45.0gϡ���������ʵ�����Ϊx�����ɶ�����̼������Ϊy

![]()
![]()
![]() y=3.96g
y=3.96g
�������������������![]()
���������������������![]() ��
��
��3��������̼��Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ�����ŷ�Ӧ�Ľ��У�������̼�����������ӣ����������ݿ�֪��10.0g��Ʒ��45.0g����ǡ����ȫ��Ӧ�����ɶ�����̼������Ϊ3.96g����10.0g��Ʒ��50.0g�����Ϻ����������̼������������Ĺ�ϵͼΪ��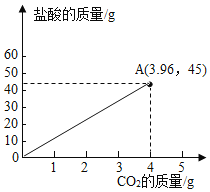

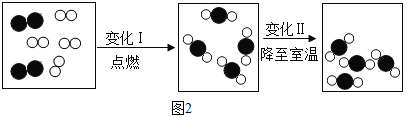
����Ŀ�����������еij���Ԫ��,��ȱ�ƶ����¹������ɡ����Ͳ��ȵĻ���Ӧ��ҽ����ָ���·��ø�Ƭ��ij����ҩ��˵����IJ�����Ϣ��ͼ1��ʾ���ֽ�100g���� (HCI) �ֳ���ȷݣ���μӵ���40Ƭ��ҩ���Ƴɵķ�ĩ��(�����ɷֲ������ᷴӦ)���õ������������ϵͼ��ͼ2.������й���Ϣ�ش����⡣(��֪��Ӧ�Ļ�ѧ����ʽΪ:![]() )
)
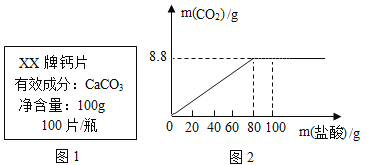
ʵ����� | ��һ�� | ������ | ���Ĵ� | ����� |
�������������(g) | 20 | 20 | 20 | 20 |
ʣ����������(g) | 35 | a | 20 | b |
(1)������a����ֵΪ ;b����ֵΪ ��
(2)��Ʒ�Ʋ���ҩ����CaCO3�������������Ƕ���? (д��������̣� ��������ȷ��0.1%)
(3)��ij���貹��0.4�˸�Ԫ�أ�����������ֲ��Ƽ� Ƭ�� (�������ʲ�����Ԫ��)
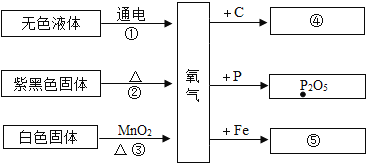
����Ŀ���ס��ҡ����������ʵ�ת����ϵ����ͼ��ʾ����������ʾ��Ӧһ��ʵ�֣��������ʺͷ�Ӧ��������ȥ��������ѡ���ʵ��ͼʾת������
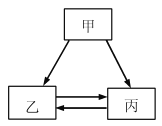
ѡ�� | �� | �� | �� |
A | H2SO4 | H2 | H2O |
B | C | CO | CO2 |
C | Ca(OH)2 | CaCl2 | CaCO3 |
D | NaOH | NaCl | NaNO3 |
A. A B. B C. C D. D