��Ŀ����
����Ŀ�����������������벻����ѧ��
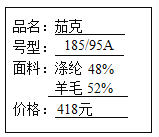
��1����ͼ��ijƷ�Ʒ�װ��ǩ�ϵIJ������ݣ�������������Ȼ��ά����_________�������г���_________�ķ�������ϳ���ά����Ȼ��ά��
��2�������������________________����ȥˮ���е�ˮ��[ˮ������Ҫ�ɷ���CaCO3��Mg(OH)2]��
��3������ϴ�ྫ��ȥ�;��ϵ����ۣ�����Ϊϴ�ྫ�����۾���___________���á�
��4������������ζ�������ͣ�������ͬѧ���Ż�û���ǵĿ��֣��ܻؽ��ң���ƿ�Ǻ���ˮ��ӿ���������û�ѧ����ʽ������ԭ��:____________��
��5������ʳƷ�и�����Ӫ������Ϊ�����ṩ��������___________������ţ���
a���� b��Ȫˮ c�߲� dţ���
���𰸡���ë ���� ʳ�� �黯 H2CO3=H2O+CO2�� ad
��������
��1��ͨ��ͼʾ���Կ������������У���ë������Ȼ��ά�����������л��ϳɲ��ϡ���ë�ijɷ��ǵ����ʣ���ȼ���ս���ë����ζ������������û���ս���ë����ζ���ʿ���ͨ�����յķ�������ϳ���ά����Ȼ��ά��
��2��ˮ������Ҫ�ɷ���CaCO3��Mg(OH)2�������ᷴӦ���������ó����е�ʳ����ϴˮ���е�ˮ����
��3��ϴ�Ӽ������黯���ã��ܳ�ȥ�����ϵ����ۣ�
��4����ˮ�ǽ�������̼�����ѹ֮���Ƴɵģ�����ˮƿ�ǣ�ѹǿ��С��������̼���ܽ�ȼ�С�����д������ݴ�ƿ���ݳ���˵����������ܽ����ѹǿ�ļ�С����С����ѹǿ�����������Ӧ�Ļ�ѧ����ʽ��H2CO3=H2O+CO2����
��5�����ࡢ֬���������ʶ���Ϊ�����ṩ�����������и����е����ʣ���Ϊ�����ṩ�������߲��и�����ά���أ�����Ϊ�����ṩ������ţ����и����е����ʣ���Ϊ�����ṩ��������Ȫˮ����Ϊ�����ṩ��������ѡad��

 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�����Ŀ�����Ϲ���63���������������ѧ���������������δ����������������������ʳ������ס�������������뻯ѧ������ء�
��1�����з�װ��Ҫ���л��ϳɲ����Ƴɵ���_______�����ţ���
A ��ë�� B ��������ȹ C �����˶��� D ����T��
��2���ٶ���������ϲ����ʳƷ������Ҫ�ɷ������ʾ
��Ŀ | ˮ | ������ | ��֬ | ���� | ά����B1 | ά����B2 | �� | �� |
ÿ100�� | 89.30 g | 4.70 g | 1.30g | 2.80 g | 0.06mg | 0.03mg | 0.24 g | 1.40g |
���ϱ��п�֪���˶����к���_______ ��Ӫ���ء�
��ʳƷ��ȫ�����ܵ����ǹ�ע������ʳƷ���ж�������ʳ�õ���_______������ţ���
A �ú����ͷۣ���̼�����ƺ��л���ȣ���������ʳƷ B ù��Ĵ��� C ���������ƣ�NaNO2������ʳ�������ʳƷ D ʳ�����Ƶ���Ѽ��
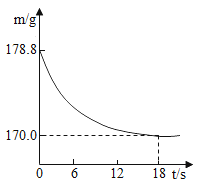
��3���Ȼ����������ȱ�ٵĵ�ζƷ��Ҳ����Ҫ�Ļ���ԭ�ϣ����ڴ����к������� MgC12��CaC12��Na2SO4�����ʣ��������㻯��������Ҫ����˱��뽫���ν��о��ơ���������ͼ��
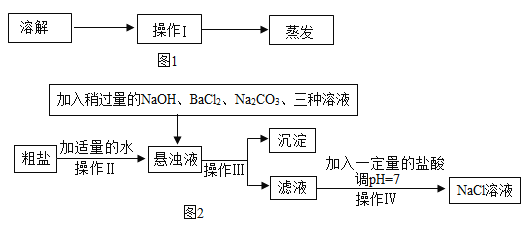
�ٲ��������õIJ��������У��ձ�����������_______��
�������������У���_______ʱ��ֹͣ���ȣ��������Ƚ���Һ���ɡ�
�ۼ����Թ�����Na2CO3��Һ�����ܳ�ȥ�����е� CaC12�⣬�������Գ�ȥ_______��
��ͨ��������������Һ�е�������_______ (�û�ѧʽ��ʾ)��
��ijͬѧ����100g��������Ϊ8.5%���Ȼ�����Һ����Ҫ��ش����⣺
�����㣩��Ҫ�Ȼ��ƹ��������Ϊ________g��
����������������ƽ�����Ȼ��ƹ���ʱ���Ȼ��ƹ���Ӧ������ƽ��_______��(����������������)��
����Ŀ��2011��5��1����������������ʵʩ���գ�ȫ�������ϸ�鴦�����Է���һ�ˣ��人������������������˾��Ϳij���������������Ρ����������еľƾ������аơ���ƺ�ơ�Ƶȣ��������Ҵ�(C2H5OH)�����ƺ��Ҵ��ɽ�������ѪҺ�У��±��������Ƽݳ����������Ƽݳ����Ľ綨����
���Ƽݳ� | 20���ˣ�100������ѪҺ�еľƾ�����<80���ˣ�100���� |
���Ƽݳ� | ѪҺ�еľƾ�������80���ˣ�100���� |
��ش��������⣺
(1)�Ҵ�(C2H5OH)�׳ƾƾ������Ҵ�������̼���⡢��ԭ�ӵĸ�����Ϊ_____��
(2)�Ҵ��������ƾ��ơ���ȼ����ȼ�ϣ����Ҵ�ȼ�յĻ�ѧ����ʽΪ______��
(3)���ƻ�ʹ�˵�____ϵͳ�����˷ܻ�������ƣ�________(��Ӱ���Ӱ��)���ļݳ����������������������������ͨ�¹ʡ�����˾��Ϳij��ѪҺ�ƾ����������Ըߴ�282���ˣ�100��������____�ݳ���
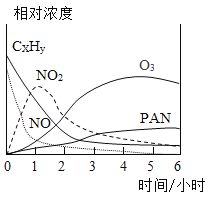
����Ŀ��ijУʵ������һƿ���õ���м����ɷ���������������ˮ��Ϊ�ⶨ���и��ɷֵ�����������ij��ȤС�鰴��ͼ��ʾװ�ý���ʵ�飨װ�����������ã��̶�װ������ȥ������������м�еijɷַ�Ӧ����ʯ���������ƺ��������ƵĻ�����
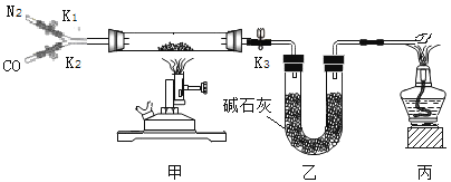
����ʵ�鲽�����£�
��������Ӳ�ʲ����ܵ�����������Ʒ����Ӳ�ʲ������У�����Ӳ�ʲ����ܺ���Ʒ��������
II�����Ӻ�װ�á�����ͨ��N2����ȼ�״��ľƾ���ƣ���Ӳ�ʲ������й�����أ���¼Ӳ�ʲ����ܺ�ʣ������������
�����ٴ����Ӻ�װ�ã�����ʵ�顣ͨ��CO,��ȼ�����ľƾ��ƺͼ״��ľƾ���ơ���Ӳ�ʲ������й�����أ�Ϩ��ƾ���ƣ�����ͨ��COֱ��Ӳ�ʲ�������ȴ���ٴμ�¼Ӳ�ʲ����ܺ�ʣ������������
ʵ�����ݼ�¼���±�:
Ӳ�ʲ����� | Ӳ�ʲ����ܺ���Ʒ | ����IIӲ�ʲ��� �ܺ�ʣ����� | ���貽����Ӳ�ʲ��� �ܺ�ʣ����� | |
���� | m1 | m2 | m3 | m4 |
��ش���������:
��1�������������ƾ��Ƶ�������____________��
��2��������Ӳ�ʲ������з�Ӧ�Ļ�ѧ����ʽΪ________��
��3����Ʒ������������������Ϊ_____����m1��m2��m3��m4�Ĵ���ʽ��ʾ��������������������û����ȫ��Ӧ����Ʒ��ˮ�������������������______������ƫ������ƫС����������������
��4���й���Ʒ������˵����ȷ����_____������ĸ��ţ���
A��Ʒ����Ԫ�ص�����Ϊ1/9(m2-m3) B��Ʒ�������ʺ��������������ܺ�Ϊm3-m1
C��Ʒ�������ʵ�����Ϊm4-m1 D��Ʒ����Ԫ�ص�����Ϊm3-m4