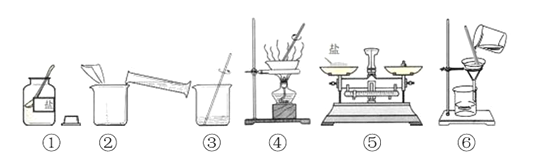
��Ŀ����
����Ŀ�����й��ڻ�ѧʵ�����Ŀ������������������������������������ȷ����
ʵ��Ŀ�� | �����Լ� | ʵ������ | ʵ����� | |
A | ����ϡ���������������Һ�Ƿ�ǡ����ȫ��Ӧ | �ڷ�Ӧ�����Һ�еμ���ɫ��̪��Һ | ���������� | ǡ����ȫ��Ӧ |
B | ����NaOH��Һ�Ƿ���� | ��������ϡ���� | �����ݲ��� | NaOH��Һ�ѱ��� |
C | ����ij�����Ƿ�̼���� | ����ϡ���� | �����ݲ��� | ������һ����̼���� |
D | �ⶨ������������������� |
| ��ȫȼ�պ��������ɼУ����뼯��ƿ��ˮ�����ԼΪ����ƿ����������֮һ | ����Լռ�������D �����֮һ(1/5) |
���𰸡�B
��������
���������A. ��̪��Һ�ı�ɫ��Χ��8.2��10.0.����ڷ�Ӧ�����Һ�еμ���ɫ��̪��Һ����������������ǡ�÷�Ӧ��Ҳ�����Ǽ��������˲���֤��ϡ���������������Һǡ����ȫ��Ӧ������B.NaOH�ڿ����л�����CO2������Na2CO3,���������ʣ������������Һ�м�������ϡ���ᣬ�ᷢ����Ӧ��Na2CO3+ 2HCl=2NaCl+H2O+ CO2�����������ݲ������������ʣ���ֻ������Ӧ��HCl+NaOH=NaCl+H2O�������ݲ�������˿��Լ���NaOH��Һ�Ƿ���ʡ���ȷ��C.��ij����������Na2SO3, ����ϡ����ᷢ����Ӧ��Na2SO3+ 2HCl=2NaCl+H2O+ SO2����Ҳ�������ݲ�������ˣ����ܵõ����ۡ�����D.��Ҫ�ⶨ������������������������ں����Ż����240 �棬�ڿ����в�����ȼ��Ӧ��ѡ���ڿ������ܹ���ȼ���Ż��͵İ��ס�����������ȫȼ�պ�Ӧ�õ���ȴ������ʱ�ٴ��ɼУ����뼯��ƿ��ˮ�����ԼΪ����ƿ����������֮һ������

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�����Ŀ�����ձ�����μ���x��Һ����������Ӧ���������ɳ�������������������x��Һ��������ϵ������ͼ��ʾ�����߱�ʾ����


��� | �ձ��е����� | x��Һ |
�� | ͭп�Ͻ� | ϡHCl |
�� | ������ϡ���� | Na2CO3��Һ |
�� | ��������� | ϡHCl |
�� | H2SO4��CuSO4��Һ | NaOH��Һ |
A���٢� B���ڢۢ� C���٢ڢ� D���ۢ�