��Ŀ����
����Ŀ����ѧ�����������ᷢչϢϢ��أ���ش�
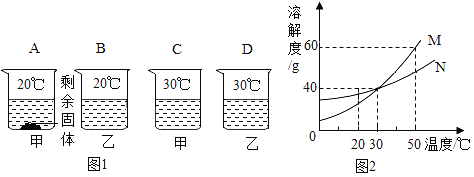
��1�����þ�ˮ����ʹ�û���̿������������______________�ԡ�
��2�������dz�����ʳƷ���ʼ�����ԭ���� ________________________________��
��3������ƿ�д��������ݳ���˵�������ܽ��___________________________��
��4��ʹ����Ȼ����ˮ��ʱҪ��֤ͨ�����ã����������о綾��_________���ѧʽ����
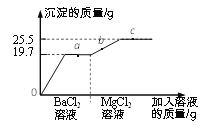
��5��Ϊ���ú�������ʣ��ɽ�̼��ˮ������Ӧ�õ����ֿ�ȼ�����壬����ʾ��ͼ���£�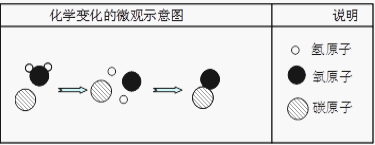
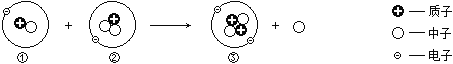
��.���������غ㶨���жϣ�������ʾ��ͼ��ȱ�ٵ�������____________����ţ���
![]()
��.�÷�Ӧ�е���С������___________________�����������ƣ���
��6�����о����ְ�����NH3��ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ��������ȼ������ˮ����һ�����嵥�ʣ�������ռ�������������ԼΪ78%������ȼ�շ�Ӧ�Ļ�ѧ����ʽ__________��
��7�������������и�������������֧��ũ���˽������أ���������Ҫ�ɷ��Ǽ��飬��������л����������������________________��ɣ���������____________���ɡ�
���𰸡� ������ �������������������ˮ������Ӧ ����ѹ�ļ�С����С CO D ̼ԭ�ӡ���ԭ�ӡ���ԭ�ӡ� 4NH3 + 3O2 ![]() 6H2O+2N2 ̼Ԫ�ء���Ԫ�أ� ������ӡ�
6H2O+2N2 ̼Ԫ�ء���Ԫ�أ� ������ӡ�
����������1�����þ�ˮ����ʹ�û���̿�����������������ԣ�����ˮ�е�ɫ�غ���ζ����2�������dz�����ʳƷ���ʼ�����ԭ�����������������������ˮ������Ӧ��������������ֹʳ���������������ʣ���3������ƿ�д��������ݳ���ƿ��������٣�ѹǿ��С��˵�������ܽ������ѹ�ļ�С����С����4����̼ȼ�������������ʱȼ�գ������һ����̼��ʹ����Ȼ����ˮ��ʱҪ��֤ͨ�����ã����������о綾��CO����5����.���������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����ࡢ����������жϣ�������ʾ��ͼ��ȱ�ٵ�����������ӡ���D����.ԭ���ǻ�ѧ�仯����С�������÷�Ӧ�е���С������̼ԭ�ӡ���ԭ�ӡ���ԭ�ӡ���6��������������ȼ����������ˮ����һ�����嵥�ʣ�������ռ�������������ԼΪ78%����������ǵ���������ȼ�շ�Ӧ�Ļ�ѧ����ʽ4NH3 + 3O2 ![]() 6H2O+2N2 ����7������������л����������������̼Ԫ�ء���Ԫ����ɣ��������ɼ�����ӹ��ɡ�
6H2O+2N2 ����7������������л����������������̼Ԫ�ء���Ԫ����ɣ��������ɼ�����ӹ��ɡ�

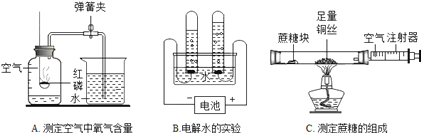
����Ŀ������ʵ���Ҳ���װ����A��Eͼ��ʾ��
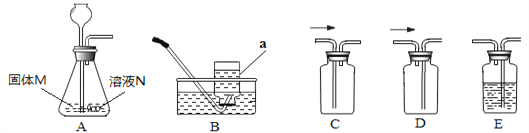
��ش��������⣺
������a������Ϊ_____________��
��������װ����ȡ���ռ���ͬ���壬��д�±��հס�
����M | ��ҺN | ��ȡ������ | �ռ�װ�� |
����ʯ | ��______________ | CO2 | ��______________ |
��__________ | ����������Һ | O2 | ��__________��C |
п�� | ϡ���� | ��____________ | B���__________ |
���ռ������CO2����Ҫ��װ��E��E��ʢװ���Լ�Ϊ__________������CO2ͨ����ɫʯ����Һ�У�������_____________��
������ͼװ��A��ȡO2ʱ������Ӧ�Ļ�ѧ����ʽΪ_____________________________��
����Ŀ��С��������е�ˮ������ˮʱ�����ֺ�����Щ��ɫ�Ĺ��塣Ϊ��̽����ɷ֣�����ȤС��ͬѧ�������Ϻ��֪��Щ��ɫ�Ĺ�����ˮ��������������Ϊˮ�к���Ca(HCO3)2��Mg(HCO3)2�ȿ����������ڼ���ʱ������CaCO3��Mg(OH)2��
��1�����н϶�Ca(HCO3)2��ˮ��_____���Ӳˮ������ˮ����������ʱ������Ӧ�Ļ�ѧ����ʽΪ__________��
��2��С����Ϊ��������˹�ص�ò����ʯ����Ϊ������ˮ���ijɷ�ֻ��CaCO3����С�ײ�ͬ�⣬��ΪҲ����ֻ��Mg(OH)2��������______________��
��3��С��Ϊ��֤���Լ��IJ��룬�������ʵ�鷽����
ʵ����� | �� �� | �� �� |
��ȡ����ˮ�����Թ��У����������_______�� | �۲쵽����_________�� ��Ӧ�Ļ�ѧ����ʽ__________�� | ˮ������CaCO3 |
����ٷ�Ӧ����Թ��еμ�2-3��NaOH��Һ | �۲쵽����__________�� | ˮ����û��Mg(OH)2�� �ҵIJ������ |
��4��С�ײ��Ͽ�С���Ľ��ۣ���Ϊ����ʵ�������ȱ�ݣ�ԭ����_________________________��