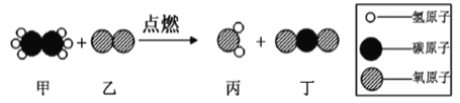
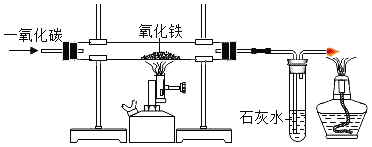
��Ŀ����
����Ŀ������һ��Ӧ�ù㷺�Ľ�����ij��ȤС��Խ�����չ����ϵ���о���
I �о����Ļ�ѧ����
��1�����ڳ����»���������Ӧ�������γ�һ�����ܵ�����Ĥ����ѧ����ʽΪ_________ �� ʵ��ǰ����Ҫ�����ı����Ƚ���______________�����������������ȤС��ͬѧ���������ú�� �����ɴ�С��ͬ��СƬ�����ں���ʵ�顣
��2�������ᡢ�����Һ�ķ�Ӧ
ʵ�� | ���� | ���� | ���� |
һ | ����Ƭ����ϡ ������ | ______________���Թܱ��� | ���������ᷢ����Ӧ����Ӧ���� |
�� | ����Ƭ������ ��������Һ�� | �����������ݲ������Թܱ� �� | ����������������Һ��Ӧ����Ӧ ���� |
�� | ������������ ͭ��Һ�� | �������к�ɫ���ʲ������� Һ�����ɫ | ������ԣ���_____ͭ |
ʵ��һ������Ϊ______________________��ʵ����������ϣ������������ơ�ˮ��Ӧ����ƫ�����ƣ�NaAlO2������������Ӧ�Ļ�ѧ����ʽΪ______________________��ʵ�������������Ľ������_____________������ڡ���С�ڡ���ͭ��
��3��С��ͬѧ��ʵ��һ�е�ϡ���ỻ�ɵ�������������Ũ����ͬ��ϡ���ᣬ��ͬʱ���� ������Ƭ������������ݽ��٣���Ӧ������
�Ա���������ʵ��������롣 ����٣�ϡ�����е������ӶԷ�Ӧ�����дٽ����á� ����ڣ�_____��
Ϊ��֤������Ƿ������Ӧ������ϡ�����м���___________������ĸ�����۲�����
A��Na2SO4 B��Na2CO3 C��NaCl
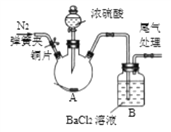
II �ⶨij����Ʒ�н���������������
������1������������������������Һ��Ӧ�������백ˮ��Ӧ��
������2��AlCl3+3NaOH=Al(OH)3��+3NaCl��AlCl3+3NH3��H2O=Al(OH)3��+3NH4Cl��
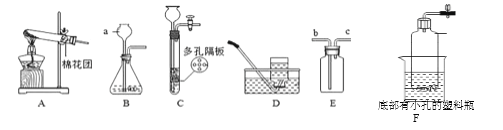
��4��С��ͬѧ��ȡ4.62gij��������Ʒ����Ʒ�����ʽ�Ϊ��������������ͼһ��ƿ�У���������ϡ��������ȫ��Ӧ������Ӧ���Һ��ֳ���Һ1����Һ2���ȷݣ��������ʵ�鷽������ͼ������ͨ�����������ⶨ��Ʒ�н�����������������
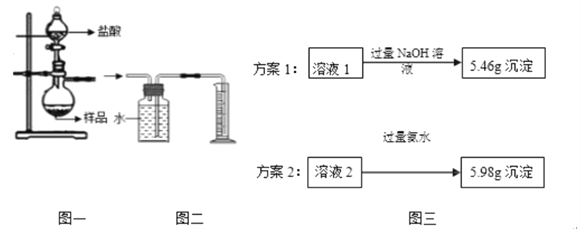
��ѡ����ȷ��һ��ʵ�鷽����������Ʒ�н�������������������д��������̣�.
��С��ͬѧ���ͼһ��ͼ��װ�ò������������ƿ�з�Ӧֹͣ��������Ͳ��ˮ������� ������ֽ���������������ƫ���ܵ�ԭ����?
���𰸡� 4Al+3O2=2Al2O3 ��ĥ�������Կɣ� �����������ݲ��� 2Al+2 NaOH + 2H2O =2NaAlO2 + 3H2�� ���� ϡ�����е���������ӶԷ�Ӧ�������������� C �����ÿ������Ԫ�ص����� 2.07g�������������Ʒ����Ԫ�ص����� 4.14g���� ���ÿ�������������� 0.51g�������������Ʒ�������������� 1.02g���� ���ÿ������������ 1.8g��������������Ϊ 77.9%�������������Ʒ���������� 3.6g��������������Ϊ 77.9%�� ��Ӧ���ȣ�����ƿ������������ͣ��ų���ˮƫ��
����������1���������������ڳ����·�Ӧ������������𣻸���Ҫ���������������������ȥ�����2���������Ļ�Ա�ǿ�������������ơ�ˮ��Ӧ����ƫ�����ƺ�������𣻸������Ļ�Ա�ͭǿ�����3������ϡ�����ϡ������������Ӳ�ͬ���������4���٢����ݷ�Ӧ�ų��������������1�����������ڳ����·�Ӧ��������������Ӧ�Ļ�ѧ����ʽΪ 4Al+3O2=2Al2O3��ʵ��ǰ����Ҫ�����ı����Ƚ��д�ĥ��������2�����Ļ�Ա�ǿ����ʵ��һ������Ϊ�����������ݲ����������������ơ�ˮ��Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����ʵ�������������Ľ�����Դ���ͭ����3������ڣ�ϡ�����е���������ӶԷ�Ӧ�������������ã�Ϊ��֤������Ƿ������Ӧ������ϡ�����м���C���۲�������4����������Ʒ����Ԫ�ص�����=5.98g��![]() ��2=4.14g��������Ʒ��������������=��4.62-4.14����
��2=4.14g��������Ʒ��������������=��4.62-4.14����![]() =1.02g��������Ʒ����������=4.62g-1.02g=3.6g����Ʒ�н���������������=
=1.02g��������Ʒ����������=4.62g-1.02g=3.6g����Ʒ�н���������������=![]() ��100��=77.9������С��ͬѧ���ͼһ��ͼ��װ�ò������������ƿ�з�Ӧֹͣ��������Ͳ��ˮ������� ������ֽ���������������ƫ���ܵ�ԭ���Ƿ�Ӧ���ȣ�����ƿ������������ͣ��ų���ˮƫ�ࡣ
��100��=77.9������С��ͬѧ���ͼһ��ͼ��װ�ò������������ƿ�з�Ӧֹͣ��������Ͳ��ˮ������� ������ֽ���������������ƫ���ܵ�ԭ���Ƿ�Ӧ���ȣ�����ƿ������������ͣ��ų���ˮƫ�ࡣ

����Ŀ����������ͼ����Ϣ��ѧϰ��һ����Ҫ�������±����Ȼ��ƺ�������ڲ�ͬ�¶��µ��ܽ�ȡ�
�¶�/�� | 20 | 40 | 60 | 80 | |
�ܽ��/g | NaCl | 36.0 | 36.6 | 37.3 | 38.4 |
KNO3 | 31.6 | 63.9 | 110 | 169 | |
�����ֹ���������ˮ�е��ܽ��������ͼ��ʾ:
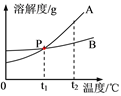
����ϸ�Ķ�ͼ����ش��������⣺
��1��A���߱�ʾ����________���ܽ�����ߡ�(�NaCl����KNO3��)
��2��P������___________________________��
��3��t2��ʱ������������A��B�ı�����Һ������t1�棬������Һ��������С��ϵ��A____B�����<������>����=����