��Ŀ����
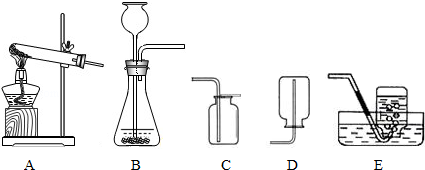
ͼ1��ʵ���ҳ��õ�װ�ã���ش��������⣺
��1��ʵ����������غͶ������̻����ȡ�����ķ���װ������ ��������ţ�����Ӧ�Ļ�ѧ����ʽΪ�� ����
��2��д��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼�Ļ�ѧ����ʽ�� �����ռ�CO2Ӧѡ�õ�װ������ ��������ţ���
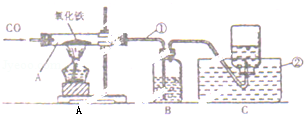
��3������ͼ2��ʾװ����H2����������H2�������ͨ����Һ©������һ�������ϡ���ᣬ��Բ����ƿ��ʢ�ŵ�0.65gп����ַ�Ӧ��ϡ������������
��װ�ýӿڵ�����˳��Ϊ��a���� ����d���b��c����c��b������
��������0.65gп������ϡ���ᷴӦ�ڸ�������Ӧ���ռ���224mL��H2����3��ʵ�������H2�����ƽ��ֵԼΪ239mL������ʵ�������ȷ���淶����ɸ�������Ҫԭ������ ����
��1��A�� 2KClO3 2KCl+3O2���� ��2��CaCO3+2HCl�TCaCl2+H2O+CO2���� D��
2KCl+3O2���� ��2��CaCO3+2HCl�TCaCl2+H2O+CO2���� D��
��3����c��b�� �ڲ����������������ϡ��������
���������������1�����ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã����ݷ�Ӧԭ����д����ʽ��ʵ�������������ȡ������Ҫ���ȣ����ڹ�������ͣ���ѡ����װ��A��������ڶ������������������ȵ������������Ȼ��غ�����������ʽ�ǣ�2KClO3 2KCl+3O2����
2KCl+3O2����
��2������ʵ������ȡ������̼�ķ�Ӧԭ����д����ʽ���ݶ�����̼���ܶȺ��ܽ���ѡ���ռ�װ�á�ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼������ʽ�ǣ�CaCO3+2HCl��CaCl2+H2O+CO2����������̼������ˮ��������ˮ��Ӧ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���
��3������������ˮ����������ˮ���ռ����ܶȱ�ˮ���ܶ�С����������Ӷ̹ܽ��룬ˮ�ӳ���ѹ����Ͳ����ɸ�������Ҫԭ���Dz����������������ϡ����������
���㣺���鳣������ķ���װ�ú��ռ�װ����ѡȡ����

 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
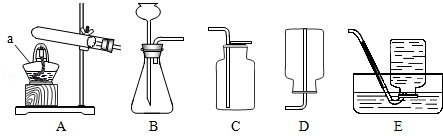
����ѧ��Ӯ�����ϵ�д���ͼ��ʵ���ҳ��õ�װ�ã������ͼ�ش��������⣺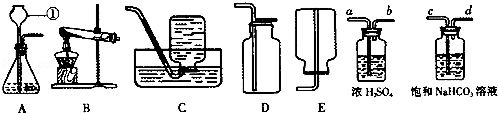
��1��д�����б�����������ƣ����� ��
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ���� ������ѡ�õķ���װ������ ��������ţ���ʵ����Ϻ�Ҫ�Ƚ����ܴ�ˮ����ȡ����Ϩ��ƾ��ƣ���ԭ������
��3���ռ�ij����ֻ�ܲ���Eװ�ã��ɴ��Ʋ��������е��������� ��
��4��ʵ�����Ƶõ�CO2�����к����Ȼ����ˮ������Ϊ�˵õ������������CO2���壬����װ�õĵ���������������˳������ ������ѡ�����ţ�
| A��a��b��c��d�� | B��b��a��c��d�� | C��c��d��a��b�� | D��d��c��b��a |