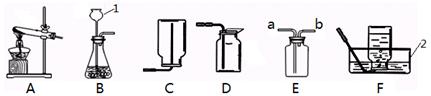
��Ŀ����
��۲�����װ�ò��ش�������⣮
��1����������c ���������� ����
��2��A װ���з�����Ӧ�Ļ�ѧ����ʽ���� ����
��3�����ø��������ȡ������Ӧѡ�õķ���װ������ �������Ӧ�ı�ţ����仯ѧ����ʽΪ�� ��������Dװ���ռ������������ķ������� ����
��4��F װ�ô�a��b ��ijһ���ӿڽ��������Դ���D��E װ���ռ����壬���ҿ��Լ�������������е��ݳ�������B��Fװ������ȡ���ռ�������̼���壬����B װ�õ�Ӧ���� ���ڣ�ѡ�a����b������
��1������©������2��CaCO3+2HCl=CaCl2+H2O+CO2����
��3��C�� 2KMnO4 K2MnO4+MnO2+O2������������ľ������ƿ�ڣ���������ľ����ȼ��������ȼ�գ���֤��������������4��a��
K2MnO4+MnO2+O2������������ľ������ƿ�ڣ���������ľ����ȼ��������ȼ�գ���֤��������������4��a��
���������������1������c������Һ��ij���©������2������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2������3�����ȸ��������ȡ�������ڹ�������ͣ���ѡ����װ��C�����ȸ��������������ء��������̺�����������ʽ�ǣ�2KMnO4 K2MnO4+MnO2+O2��������Dװ���ռ������������ķ����ǽ������ǵ�ľ�����ڼ���ƿ�ڣ��۲��Ƿ�ȼ������������4��������̼���ܶȱȿ�����Ӧ�ӳ����ܽ�������������ѹ������ƿ�ϲ��ų���
K2MnO4+MnO2+O2��������Dװ���ռ������������ķ����ǽ������ǵ�ľ�����ڼ���ƿ�ڣ��۲��Ƿ�ȼ������������4��������̼���ܶȱȿ�����Ӧ�ӳ����ܽ�������������ѹ������ƿ�ϲ��ų���
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ������