��Ŀ����
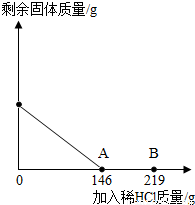
| ��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״�� ��������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⡣Ȼ������ ����μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺ |
 |
| ��1��������ϡ����������ͼ��A��ʱ����������Һ�����ʵ����������� ��2��������ϡ����������ͼ��B��ʱ���������ձ��м���������ĩ״��������ո������ݲ��������ʱ�ձ��и�Ԫ�������� |
|
(1) 146 g��10%=14.6 g |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
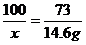 x=20g
x=20g y=22.2 g
y=22.2 g 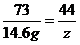 z=8.8 g
z=8.8 g 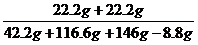 ��100%=15%
��100%=15% 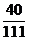 ��100%��24g
��100%��24g ��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺ ��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺
��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺ ��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��
��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ�� ��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺