��Ŀ����
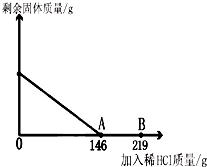
��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺��1��������ϡ����������ͼ��A��ʱ����������Һ�����ʵ�����������
��2��������ϡ����������ͼ��B��ʱ���������ձ��м���������ĩ״��������ո������ݲ��������ʱ�ձ��и�Ԫ��������

���𰸡���������1��������ϡ����������ͼ��A��ʱ��̼��ƺ�ϡ����ǡ����ȫ��Ӧ��������ҺΪ�Ȼ�����Һ�����ݻ�ѧ����ʽ
����Ȼ��Ƶ���������һ�������������Һ�����ʵ�����������
��2������ͼ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״�����ǡ����146gϡ���ᷴӦ���ɵ�219gϡ�������ܷ�Ӧ��̼��ƺ��Ȼ��Ƶķ�ĩ״���������������������غ㶨��̼��ƺ��Ȼ����и�Ԫ�ص�����֮�ͼ�Ϊ�ձ��и�Ԫ��������
����⣺��ԭ�������̼��Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy�����ɵĶ�����̼������Ϊz
CaCO3 +2HCl=CaCl2 +H2O+CO2��
100 73 111 44
x 146g×10% y z
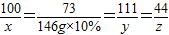
x=20g
y=22.2g
z=8.8g
��1��������ϡ����������ͼ��A��ʱ����������Һ�����ʵ���������Ϊ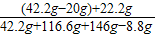 ×100%=15%
×100%=15%
��2����219gϡ�������ܷ�Ӧ��̼��ƺ��Ȼ��Ƶķ�ĩ״����������Ϊa
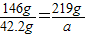
a=63.3g
��Ϊ42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״������к���20g̼��ƺͣ�42.2g-20g���Ȼ��ƣ�
��63.3g̼��ƺ��Ȼ��Ƶķ�ĩ״������к���30g̼��ƺͣ�63.3g-30g���Ȼ��ƣ�
�ձ��и�Ԫ�ص�����Ϊ��30g×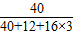 +��63.3g-30g��×
+��63.3g-30g��×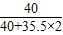 =24g
=24g
��1��������ϡ����������ͼ��A��ʱ��������Һ�����ʵ���������Ϊ15%��
��2��������ϡ����������ͼ��B��ʱ����ʱ�ձ��и�Ԫ������Ϊ24g��
�����������ѶȽϴ���Ҫ�������Ի�ѧ����ʽ����Ϊ������ͬʱ������Һ�ȷ���ļ����⣬��������Ŀһֱ���п����ȵ㣬��Ҫ����ѧ�����ۺϷ��������ͼ���������
����Ȼ��Ƶ���������һ�������������Һ�����ʵ�����������
��2������ͼ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״�����ǡ����146gϡ���ᷴӦ���ɵ�219gϡ�������ܷ�Ӧ��̼��ƺ��Ȼ��Ƶķ�ĩ״���������������������غ㶨��̼��ƺ��Ȼ����и�Ԫ�ص�����֮�ͼ�Ϊ�ձ��и�Ԫ��������
����⣺��ԭ�������̼��Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy�����ɵĶ�����̼������Ϊz
CaCO3 +2HCl=CaCl2 +H2O+CO2��
100 73 111 44
x 146g×10% y z
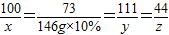
x=20g
y=22.2g
z=8.8g
��1��������ϡ����������ͼ��A��ʱ����������Һ�����ʵ���������Ϊ
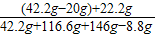 ×100%=15%
×100%=15%��2����219gϡ�������ܷ�Ӧ��̼��ƺ��Ȼ��Ƶķ�ĩ״����������Ϊa
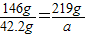
a=63.3g
��Ϊ42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״������к���20g̼��ƺͣ�42.2g-20g���Ȼ��ƣ�
��63.3g̼��ƺ��Ȼ��Ƶķ�ĩ״������к���30g̼��ƺͣ�63.3g-30g���Ȼ��ƣ�
�ձ��и�Ԫ�ص�����Ϊ��30g×
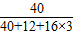 +��63.3g-30g��×
+��63.3g-30g��×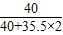 =24g
=24g��1��������ϡ����������ͼ��A��ʱ��������Һ�����ʵ���������Ϊ15%��
��2��������ϡ����������ͼ��B��ʱ����ʱ�ձ��и�Ԫ������Ϊ24g��
�����������ѶȽϴ���Ҫ�������Ի�ѧ����ʽ����Ϊ������ͬʱ������Һ�ȷ���ļ����⣬��������Ŀһֱ���п����ȵ㣬��Ҫ����ѧ�����ۺϷ��������ͼ���������

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
�����Ŀ
 ��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺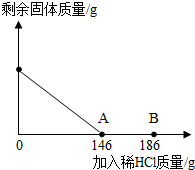 ��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺
��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺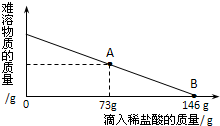 ��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��
��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��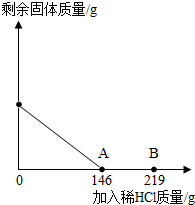 ��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺