��Ŀ����
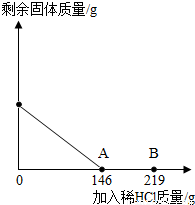
 ��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��
��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ�����������ش��������⣺
��1���ڵ���ϡ����Ĺ����У��۲쵽������ʵ�������ǣ�
��
���岻���ܽ�
���岻���ܽ�
�����������
���������
����2��������10%��ϡ������ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�д��ѧʽ��
CaCl2
CaCl2
����3��������10%��ϡ����146gʱ����B�㣩����ͨ�����㣬���ʱ�ձ������ò�������Һ������������������ȷ��0.1g��
��������1������̼��ƺ�ϡ���ᷴӦ�������н��
��2������̼��ƺ�ϡ���ᷴӦ�ķ���ʽ������ĩ״��������ɣ����з������
��3�����ݻ�ѧ��Ӧ�ķ���ʽ�������������������������������غ㶨�ɼ�������ձ������ò�������Һ��������
��2������̼��ƺ�ϡ���ᷴӦ�ķ���ʽ������ĩ״��������ɣ����з������
��3�����ݻ�ѧ��Ӧ�ķ���ʽ�������������������������������غ㶨�ɼ�������ձ������ò�������Һ��������
����⣺
��1������̼��ƺ�ϡ���ᷴӦ������֪�ڵ���ϡ����Ĺ����У��۲쵽������ʵ�������ǣ����岻���ܽ⣻
����������� �ʴ�Ϊ�����岻���ܽ⣻ �����������
��2������̼��ƺ�ϡ���ᷴӦ�ķ���ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O��������10%��ϡ������ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�CaCl2�� �ʴ�Ϊ��CaCl2��
��3��B��ʱ����ϡ���������Ϊ��146g��10%=14.6g
������CO2������Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
73 44
14.6g x
=
��� x=8.8g
�ձ������ò�������Һ������Ϊ��188.8g+42.2g+146g-8.8g=368.2g��
���ձ������ò�������Һ������Ϊ368.2g��
��1������̼��ƺ�ϡ���ᷴӦ������֪�ڵ���ϡ����Ĺ����У��۲쵽������ʵ�������ǣ����岻���ܽ⣻
����������� �ʴ�Ϊ�����岻���ܽ⣻ �����������
��2������̼��ƺ�ϡ���ᷴӦ�ķ���ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O��������10%��ϡ������ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�CaCl2�� �ʴ�Ϊ��CaCl2��
��3��B��ʱ����ϡ���������Ϊ��146g��10%=14.6g
������CO2������Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
73 44
14.6g x
| 73 |
| 44 |
| 14.6g |
| x |
�ձ������ò�������Һ������Ϊ��188.8g+42.2g+146g-8.8g=368.2g��
���ձ������ò�������Һ������Ϊ368.2g��
��������Һ�ͻ�ѧ��Ӧ�ںϵ���Ŀ�����ۺ��Ե����ͣ�Ҫ��Ƚϸߣ�ͨ��ѧ���ڼ���ʱ�����������ʵ�ʲμӷ�Ӧ������Һ�����ʵ����������Ҫ��ѧ���㹻ϸ�ġ��������������������

��ϰ��ϵ�д�
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
 ��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺ ��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺
��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺ ��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺