��Ŀ����
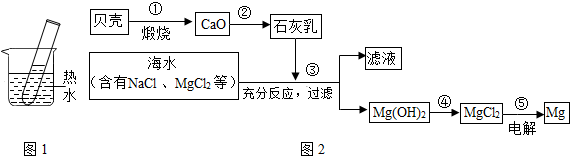
������һ�������Դ�⣬���ǿ��ԴӺ�ˮ����ȡʳ�Σ����Դ�Ϊԭ���Ƶþ��й㷺��;���ռ����ȡ���������£�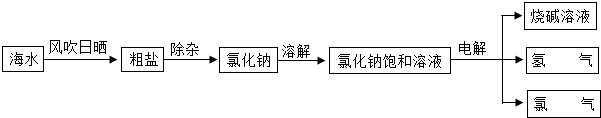
��1�����÷紵��ɹ���ԴӺ�ˮ����ȡ���Σ��紵��ɹ����Ҫ������
��2��Ҫ��ȥʳ��ˮ�л��е���ɳ������������
��3���û�����������Ʒ�����������������ã�������������Cl?2����ȼ�������Ȼ������壬�÷�Ӧ�Ļ�ѧ����ʽ��
��4��С��ͬѧ��һֻ���ʵ������������ռ����Ȼ������壬����������ˮ�ܷ�������������ޱ���ˣ���ԭ����
��5���ڹ�ҵ�����У�ԭ���ϲ�����ȫ��ת��Ϊ�ɲ�Ʒ��С��ͬѧ�Ӹû������IJֿ��й���һƿ�ռС��˵�������ռ�������ܱ��ʣ���С������ʮ���ɻ���ͬѧ�����о����������ʵ�鷽����������ʵ�飬������գ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ������ˮ���μӹ�����ϡ���� | ��Ʒ��һ������ | |
| ����������Һ�еμ� |
��Ʒ��һ������NaCl |
��������1������ɹ�ε�ԭ���������ɣ�
��2�����ݻ�������ķ���������
��3�����������ٽ��Ԫ���غ�������ɣ�
��4��������Ŀ��Ϣ��ȼ�յĸ�����з�����������
��5�������������տ����еĶ�����̼����̼���ƶ����ʣ�
ʵ�鷽�����Ǽ���̼���ƵĴ��ڣ�ʵ�鷽�����Ǽ����Ȼ��ƵĴ��ڣ�
��2�����ݻ�������ķ���������
��3�����������ٽ��Ԫ���غ�������ɣ�
��4��������Ŀ��Ϣ��ȼ�յĸ�����з�����������
��5�������������տ����еĶ�����̼����̼���ƶ����ʣ�
ʵ�鷽�����Ǽ���̼���ƵĴ��ڣ�ʵ�鷽�����Ǽ����Ȼ��ƵĴ��ڣ�
����⣺
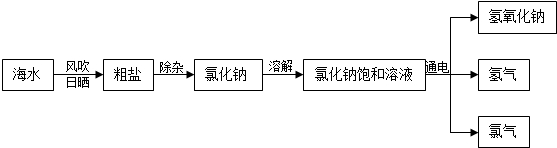
��1���Ӻ�ˮ����ȡʳ�β��������ᾧ�ķ������紵��ɹ��Ϊ�˼���ˮ�ֵ��������ʴ�Ϊ������ˮ�֣�
��2����Ϊ��ɳ������ˮ�����Ȼ�������ˮ�����Կ��Բ��ù��˵ķ�����ȥ��ɳ���ʴ�Ϊ�����ˣ�
��3���������⣺����Ȼ�����Һ���Եõ�����������Һ�����������������Ե���Ȼ��Ƶķ���ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2��
��4���������⣺������������ȼ�������Ȼ��⣬���Է���ʽΪ��H2+Cl2
2HCl���ɼ�ȼ�ղ�һ����Ҫ�����μӣ��ʴ�Ϊ��H2+Cl2
2HCl��ȼ�ղ�һ���������μӣ�
��5�������������տ����еĶ�����̼����̼���ƶ����ʣ�
ʵ�鷽�����Ǽ���̼���ƵĴ��ڣ��ܽ��ϡ��������������ݣ�˵����Ʒ�к���̼���ƣ�ʵ�鷽�����Ǽ����Ȼ��ƵĴ��ڣ���AgNO3��Һ��
�а�ɫ�������ɣ�˵�������Ȼ��ƣ�
�ʴ�Ϊ��
��1��ʹˮ��������������ˮ��?
��2������?
��3��H2+Cl2
2HCl ȼ�ղ�һ��Ҫ�������IJ��루����������ȼ�ԣ�?
��4���Ȼ������弫������ˮ����������ѹǿ��С��2Al+6HCl=2AlCl3+3H2��
?��5��ʵ�鲽�裺��AgNO3 ʵ�������д������ݲ��� Na2CO3����AgNO3 ʵ�������а�ɫ��������
��1���Ӻ�ˮ����ȡʳ�β��������ᾧ�ķ������紵��ɹ��Ϊ�˼���ˮ�ֵ��������ʴ�Ϊ������ˮ�֣�
��2����Ϊ��ɳ������ˮ�����Ȼ�������ˮ�����Կ��Բ��ù��˵ķ�����ȥ��ɳ���ʴ�Ϊ�����ˣ�
��3���������⣺����Ȼ�����Һ���Եõ�����������Һ�����������������Ե���Ȼ��Ƶķ���ʽΪ��2NaCl+2H2O
| ||
��4���������⣺������������ȼ�������Ȼ��⣬���Է���ʽΪ��H2+Cl2
| ||
| ||
��5�������������տ����еĶ�����̼����̼���ƶ����ʣ�
ʵ�鷽�����Ǽ���̼���ƵĴ��ڣ��ܽ��ϡ��������������ݣ�˵����Ʒ�к���̼���ƣ�ʵ�鷽�����Ǽ����Ȼ��ƵĴ��ڣ���AgNO3��Һ��
�а�ɫ�������ɣ�˵�������Ȼ��ƣ�
�ʴ�Ϊ��
��1��ʹˮ��������������ˮ��?
��2������?
��3��H2+Cl2
| ||
��4���Ȼ������弫������ˮ����������ѹǿ��С��2Al+6HCl=2AlCl3+3H2��
?��5��ʵ�鲽�裺��AgNO3 ʵ�������д������ݲ��� Na2CO3����AgNO3 ʵ�������а�ɫ��������
��������ȼ���������п���ȼ�գ���ȼ�ղ�һ����Ҫ���������μӣ��磺H2+Cl2
2HCl��ͬѧ����Ҫע��!
| ||

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ