��Ŀ����
С��ѧ�˻�ѧ֪�������������Ե������������ȣ��������ʣ�ʵ������һƿ������Һ��С�������������������������Һ����ȡһ�ྻС�ձ�����������Ϊ18.2g��Ȼ�������е�������������Һ�������������Ϊ53.5g��֮��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣩�����С�ձ��з�Ӧ�����������治�������ݲ������ٴγ�����������Ϊ64.2g��
��ش��������⣺
��1����Ӧ�в���������������� ��
��2���������Ƶ�����������Һ�����������Ƕ��٣���д��������̣�
��3����Ҫʹ������Һ������������Сһ������Ӧ������ٿ�ˮ����д��������̣�
��ش��������⣺
��1����Ӧ�в����������������
��2���������Ƶ�����������Һ�����������Ƕ��٣���д��������̣�
��3����Ҫʹ������Һ������������Сһ������Ӧ������ٿ�ˮ����д��������̣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,��ˮϡ�ı�Ũ�ȵķ���,�й��������������ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���
��������Ӧǰ��������Ϊ��Ӧ����������������
�����������������Լ��㷴Ӧ����������������������һ�����Լ������Ƶ�����������Һ������������
��Һϡ��ǰ�������������䣮
�����������������Լ��㷴Ӧ����������������������һ�����Լ������Ƶ�����������Һ������������
��Һϡ��ǰ�������������䣮
����⣺��1����Ӧ��������������Ϊ��53.5g+10.8g-64.2g=0.1g��
���0.1g��
��2���跴Ӧ������������������Ϊx��
Fe+H2SO4�TFeSO4+H2����
152 2
x 0.1g
=
��
x=7.6g��
���Ƶ�����������Һ����������Ϊ��
��100%=15.8%��
�����Ƶ�����������Һ����������Ϊ16.5%��
��3����Ӧ�ü���ˮ������Ϊy��
��64.2g-18.2g+x����7.9%=7.6g��
x=48g��
��Ӧ�ü���48gˮ��
���0.1g��
��2���跴Ӧ������������������Ϊx��
Fe+H2SO4�TFeSO4+H2����
152 2
x 0.1g
| 152 |
| x |
| 2 |
| 0.1g |
x=7.6g��
���Ƶ�����������Һ����������Ϊ��
| 7.6g |
| 64.2g-18.2g |
�����Ƶ�����������Һ����������Ϊ16.5%��
��3����Ӧ�ü���ˮ������Ϊy��
��64.2g-18.2g+x����7.9%=7.6g��
x=48g��
��Ӧ�ü���48gˮ��
������������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
��NH4NO3����Ʒ������ĵ�������������36%���ʿ��ܻ���������� ��������
| A��CO��NH2��2 |
| B��C3H6N6 |
| C����NH4��2CO3 |
| D��NH4Cl |
��ѧ��Ӧǰ�����п��ܸı���ǣ�������
| A�����Ӹ��� | B��ԭ����Ŀ |
| C���������� | D��ԭ������ |
�������ӽṹʾ��ͼ�У���ʾ�����ӵ��ǣ�������
A�� |
B�� |
C�� |
D��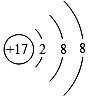 |
 ��1������������ʢ����һ��һ����֧ȼ�ŵ�������ձ��е��������̼�����ֵ��������²��������ϲ��������Ϩ�𣮵�������ձ���������֧ȼ�ŵ������ϣ���ͼ�����������Σ�
��1������������ʢ����һ��һ����֧ȼ�ŵ�������ձ��е��������̼�����ֵ��������²��������ϲ��������Ϩ�𣮵�������ձ���������֧ȼ�ŵ������ϣ���ͼ�����������Σ� ij��ѧС����������װ�ã�����װ������ȥ������������ʵ��̽����
ij��ѧС����������װ�ã�����װ������ȥ������������ʵ��̽����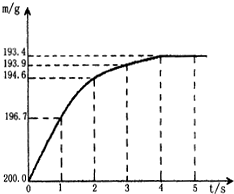 ��ʢ�к����ʵ�̼�����ƹ��壨NaHCO3��20.0g���ձ��У�����һ������ϡ������Һǡ�÷�Ӧ�����ʲ���Ӧ������Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ202.2g����Ӧ�Ļ�ѧ����ʽΪ��
��ʢ�к����ʵ�̼�����ƹ��壨NaHCO3��20.0g���ձ��У�����һ������ϡ������Һǡ�÷�Ӧ�����ʲ���Ӧ������Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ202.2g����Ӧ�Ļ�ѧ����ʽΪ��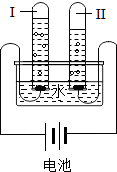 ʵ���⣺����ͼ���Թܢ��е�������
ʵ���⣺����ͼ���Թܢ��е������� ��ϡ���������ͼ��ʾʢ�в�ͬ���ʵ��Թ��У�
��ϡ���������ͼ��ʾʢ�в�ͬ���ʵ��Թ��У�