��Ŀ����
����Ŀ����1��ˮ�Ժ���Ա������dz���Ҫ��Ŀǰ��������ˮ����Դ������
A.�ӵ���Я��ˮ
B.������ȼ�ϵ���ṩˮ��������������һ�������·�����Ӧ��Ϊ�ɴ��ṩ������ͬʱ����ˮ����д����ػ�ѧ����ʽ��_______________��
C.ͨ������������������Һ�е� ˮ�ͺ���������ˮ�����ռ��������������������������������У����ӵ�����_________����ı䡱���䡱����
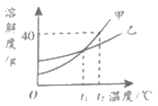
��2����ҳ���������̲����Ҳ��е���Ȼ��������Ϊδ����Դ�ġ����ǡ�����Ȼ������__________����ɡ����ɡ���������Դ������Ҫ�ɷֵĻ�ѧʽΪ___________��Ϊ������ԴΣ��������������������������Դ����̫���ܡ�___________��___________�ȡ�
���𰸡� 2H2+O2![]() 2H2O ���� ���� CH4 ���� ���ܻ�����ܻ����ܻ�ϫ�ܵ�
2H2O ���� ���� CH4 ���� ���ܻ�����ܻ����ܻ�ϫ�ܵ�
����������1��B.������ȼ�ϵ���ṩˮ����������Ϊȼ�գ�����������������Ӧ����ˮ�����Ի�ѧ����ʽΪ��2H2+O2 ![]() 2H2O��
2H2O��
C. �������������������������仯�����ӵ�����䣻
��2����Ȼ�����ڻ�ʯȼ�ϣ����ڲ���������Դ������Ҫ�ɷ��Ǽ��飬��ѧʽΪCH4������������������������Դ��̫���ܡ����ܡ����ܡ������ܡ����ܡ���ϫ�ܵȡ�

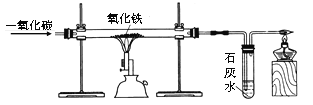
 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ѧС����Ӣ����ѧ�ҷ����ڡ�����Ļ�ѧʷ�����������������ȼ�չ��̽�������һ��̽����

���������ϡ�
����ͭ(CuSO4)��һ�ְ�ɫ��ĩ������ˮ����ˮ���Ϊ��ɫ��
������ʵ�顿
��� | ʵ�鲽�� | ʵ������ | ʵ����� |
I |
| ���洦����ɴ�����ֺ��ȵĻ������漰���Ĵ�����ɴ�������Ա仯 | |
II |
| �������ĵĵ������������̣� | ���Ĵ��п�ȼ������ |
III |
| ����ȼ�յIJ�������ˮ�Ͷ�����̼ |
(1)ʵ��I�õ��Ľ�����_________________________________________________��
(2)��ȫʵ��II��ʵ������______________________________________________��
(3)ʵ��III�У�����ͭ��ĩ��������___________________���õ�������ȼ�յIJ����к��ж�����̼����һ��������Ӧ��ʵ��������___________________________��
(4)����ʵ��III�����Եõ����������Ԫ����������________________________��
����˼�����ۡ�
(5)ʵ��III�Ǹ�С��ͬѧ����ͼ��ʾʵ��ĸĽ�������ͼ��ʾʵ����ȣ�ʵ��3���ŵ���______��

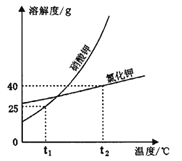
����Ŀ�����п���ѧʵ�鿼���У�С��ͬѧ�鵽����Ŀ�ǡ��������к�����������Һ����
��1��С����ɸ�ʵ��IJ��ֲ�����������ͼ��ʾ��

��С���������������������Դ������____________������ĸ��ţ���
�ڲ���B�У�������̪��________ɫ������C�е�����________________________����ʱ˵���������������ᷢ�����кͷ�Ӧ,��Ӧ����ʽΪ________________________________
��2��ʵ�鿼�������ѧ��ȤС���ͬѧ����ʦһ������ʵ���ҡ���������Һ��ʱ�����ַ�Һ���е���ҺΪ��ɫ��С��ͬѧ�������ҺΪ���ԡ�С����ͬ�⣬����Ϊ��ҺҲ���������ԣ�������________________��
��3��������ʵ��̨ʱ�������ֿ�����һ������г�ġ�����������ͼ����

�ٴ���˾��������뵽���������ܱ����ˣ��ñ��ʷ�Ӧ��_____________________
��Χ�ƴ�ƿNaOH��Һ�Ƿ���ʵ����⣬��ȤС��ͬѧ���õ�ʱʵ�����ϵ������Լ����Ȼ�����Һ��ϡ���ᡢ��̪��Һ��չ����̽�����
С��ȡ������Һ���Թ��У��μ�ij���Լ��������ݲ�����֤��NaOH��Һ�Ѿ����ʡ�����ΪС�����ӵ��Լ���_______________
С����֤�����ʵ���Һ���д�NaOH���������С���������̽��������
̽��Ŀ�� | ̽������ | Ԥ������ |
������Һ�е�CO32�� | �٣�ȡ������Һ���Թ��У��μ�������__________�Լ� | �а�ɫ�������� |
֤����Һ���д�NaOH | �ڣ���ʵ���������Һ�еμӷ�̪��Һ | ____________ |
ͨ������̽����˵������������Һ��¶�ڿ��������ױ��ʣ���Ӧ�ܷⱣ�档