��Ŀ����
����Ŀ��ӵ�С����������̡�ˮ���������ü���ÿ���й��˵����롣
��������Ҫ�ྻ�Ŀ����������еij�����O3�������������ߣ��������Ϊͬ���������������_______�����в������ӿ�����PM2.5����_______�����ţ���
A. ¶��������� B. ���������ﳾ C. ��չ�����ͨ����̼����
��������Ҫ������Դ��Ŀǰ��������������Դ��ú��_______����Ȼ���Ȼ�ʯȼ�ϡ���ʯȼ�ϲ���������������Ϊ���������Դ����Ҫԭ��������ȼ�ղ�������Ⱦ������ȼ�յĻ�ѧ����ʽΪ_______����ѧ������Ŭ�����������õ�����Դ�������ܻ���_______�ȣ�дһ������
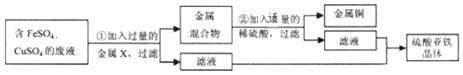
��������Ҫ�ྻ��ˮ��FeCl3Ҳ��������ˮ�����ľ�ˮ����FeCl3��ClԪ��Ϊ-1�ۣ�FeԪ�صĻ��ϼ�Ϊ_______��1mol FeCl3��Լ����_______��Fe���ӣ��ÿ�ѧ��������ʾ����
���𰸡�O2 BC ʯ�� 2H2+O2![]() 2H2O ̫���ܵȣ��������ɣ� +3 6.02��1023
2H2O ̫���ܵȣ��������ɣ� +3 6.02��1023
��������
����ͬ��Ԫ����ɵľ��в�ͬ�ṹ�����ʵĵ��ʻ���ͬ�������壬������O2���ͳ�����Ϊͬ�������壻
A��¶�����������ȼ�ջ����������С�����������PM2.5��Ⱦ��ѡ�����
B�����������ﳾ���ܼ���PM2.5��Ⱦ��ѡ����ȷ��
C����չ�����ͨ����̼���У��ܼ���PM2.5��Ⱦ��ѡ����ȷ����ѡBC��
��Ŀǰ��������������Դ��ú��ʯ�ͺ���Ȼ���Ȼ�ʯȼ�ϡ������������ڵ�ȼ�������·�Ӧ����ˮ����Ӧ�Ļ�ѧ����ʽΪ��2H2+O2![]() 2H2O������Դ�������ܻ���̫���ܵȣ�
2H2O������Դ�������ܻ���̫���ܵȣ�
��FeCl3��ClԪ��Ϊ-1�ۣ���FeԪ�صĻ��ϼ�Ϊx����x+��-1����3=0��x=+3�� 1molFeCl3����ԭ���Ӹ���Ϊ1��NA=6.02��1023��

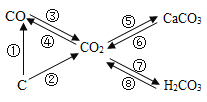
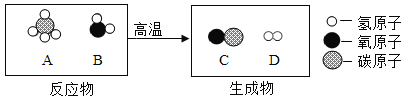
����Ŀ��������һ�����������ȷֽ⣬���ɶ�����̼��һ����̼��ˮ��ijͬѧΪ��֤����ֽ�����һ�����IJ�����ȫ�ֽ⣬��������ȫ��ͨ������װ�á�

��Aװ�������������ɫ���Ҳ���Ϊ��ɫ����д��A�з�����Ӧ�Ļ�ѧ����ʽ_______________��
��ʵ���й۲쵽B��E��ʯ��ˮ����ǣ�C��ʯ��ˮ������ǣ�˵������ֽ�IJ�������______________�����ܹ۲쵽��������___________��������Ӧ�Ļ�ѧ����ʽΪ_________________��
�۴ӻ����Ƕȿ��ǣ���θĽ���װ��___________��
��Ϊ�������ֽ����ɵ�һ����̼��������ijͬѧ������ʵ���вⶨ���������ݡ�
װ�ã�������ҩƷ�� | ��Ӧǰ������ | ��Ӧ������� |
װ��B | m1 | m2 |
װ��D�������ܼ����й��壩 | n1 | n2 |
�������������ܷ�������ֽ������һ����̼�����������ܣ���д������ʽ_________��
�����ܣ�����Ϊ����Ҫ�ⶨ��������__________����װ���в�����������Բ��ƣ�