��Ŀ����
��ѧ������������أ�
��1���ճ�������ʳ�õ��߲˺�ˮ���и�����Ӫ������________��
��2�������Ļ�ʯȼ������Ȼ����ʯ�ͺ�________�����Ƕ�����������Ӧ����һ�ֿ���������ЧӦ�����壬������Ļ�ѧʽ��________��
��3�����й��ڡ���Լ��Դ��������������������ȷ����________��
A��ֱ���ŷŹ�ҵ��ˮ���� B��ʹ�ÿɽ�������ϴ�
C��������չ�������硡�� D����չ̫���ܡ����ܵ�����Դ
��4���������˳���ʢ�����Ի����ʳ���Ϊ�������������Ƿ�����Ӧ����������������Һ��Ӧ������ƫ�����ƣ�NaAlO2����һ�ֿ�ȼ�����壮��ɸ÷�Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+________��
��5�������г��ùܵ�ú������Ҫ�ɷ�C4H10������Ȼ������Ҫ�ɷ�CH4������ȼ�ϣ�����ȫȼ�յ�������C4H10��CH4��C4H10��������������________CH4�������������������������������=������
�⣺��1���߲˺�ˮ���и�����Ӫ������ά���أ�
�ʴ�Ϊ��ά���أ�
��2�������Ļ�ʯȼ�ϰ���ú��ʯ�͡���Ȼ����������������Ӧ���ɵ����������Ƕ�����̼��
�ʴ�Ϊ��ú��CO2��
��3��ʹ�ÿɽ�������ϴ�����չ̫���ܡ����ܵ�����Դ�����ԡ���Լ��Դ��������������
�ʴ�Ϊ��BD��
��4����������������Һ��Ӧ������ƫ�����ƣ�NaAlO2����һ�ֿ�ȼ�����壬��֪2Al+2NaOH+2H2O=2NaAlO2+�����������غ��֪��������������ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��3H2����
��5����ȼ��1g�ܵ�ú������������Ϊx��ȼ��1g��Ȼ����������Ϊy��
2C4H10+13O2 8CO2+10H2O CH4+2O2
8CO2+10H2O CH4+2O2 CO2+2H2O
CO2+2H2O
106 416 16 64
1 x 1 y
 =
=
 =
=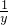
x=3.83g y=4g
������ȫȼ�յ�������C4H10��CH4��C4H10��������������C4H10�٣�
�ʴ�Ϊ����
��������1���߲˺�ˮ���и�����Ӫ������ά���أ�
��2�������Ļ�ʯȼ�ϰ���ú��ʯ�͡���Ȼ����������������Ӧ���ɵ����������Ƕ�����̼��
��3��ʹ�ÿɽ�������ϴ�����չ̫���ܡ����ܵ�����Դ�����ԡ���Լ��Դ��������������
��4�����������غ㶨����ɷ���ʽ��
��5�����ݻ�ѧ����ʽ������
���������⿼��֪ʶ��϶࣬��ʳ���Ӫ��Ԫ�ء���ʯȼ�ϡ��������塢��������������ʽ��ƽ��ȼ�ϵ�ȼ�գ�������һ�����Ѷȣ���Ҫͬѧ�ǵ�ϸ�ģ�
�ʴ�Ϊ��ά���أ�
��2�������Ļ�ʯȼ�ϰ���ú��ʯ�͡���Ȼ����������������Ӧ���ɵ����������Ƕ�����̼��
�ʴ�Ϊ��ú��CO2��
��3��ʹ�ÿɽ�������ϴ�����չ̫���ܡ����ܵ�����Դ�����ԡ���Լ��Դ��������������
�ʴ�Ϊ��BD��
��4����������������Һ��Ӧ������ƫ�����ƣ�NaAlO2����һ�ֿ�ȼ�����壬��֪2Al+2NaOH+2H2O=2NaAlO2+�����������غ��֪��������������ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��3H2����
��5����ȼ��1g�ܵ�ú������������Ϊx��ȼ��1g��Ȼ����������Ϊy��
2C4H10+13O2
 8CO2+10H2O CH4+2O2
8CO2+10H2O CH4+2O2 CO2+2H2O
CO2+2H2O106 416 16 64
1 x 1 y
 =
=
 =
=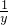
x=3.83g y=4g
������ȫȼ�յ�������C4H10��CH4��C4H10��������������C4H10�٣�
�ʴ�Ϊ����
��������1���߲˺�ˮ���и�����Ӫ������ά���أ�
��2�������Ļ�ʯȼ�ϰ���ú��ʯ�͡���Ȼ����������������Ӧ���ɵ����������Ƕ�����̼��
��3��ʹ�ÿɽ�������ϴ�����չ̫���ܡ����ܵ�����Դ�����ԡ���Լ��Դ��������������
��4�����������غ㶨����ɷ���ʽ��
��5�����ݻ�ѧ����ʽ������
���������⿼��֪ʶ��϶࣬��ʳ���Ӫ��Ԫ�ء���ʯȼ�ϡ��������塢��������������ʽ��ƽ��ȼ�ϵ�ȼ�գ�������һ�����Ѷȣ���Ҫͬѧ�ǵ�ϸ�ģ�

��ϰ��ϵ�д�
�����Ŀ

