��Ŀ����
��֪ij�Ͻ��ĩ�����⣬����������ͭ�е�һ�ֻ����֣�ij��ȤС������ʦ��ָ���£��ԺϽ��ĩ������ͭ�Ĵ������������̽�������������ϡ���������������Һ��Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2���� ����NaAlO2����ˮ����Fe��Cu��������������Һ��Ӧ��
�����롿����1���úϽ��ĩ�г����⣬����������
����2���úϽ��ĩ�г����⣬������
����3���úϽ��ĩ�г����⣬����������ͭ��
��ʵ��̽��������ʵ�����ѡ����Լ���10%���ᡢ30%NaOH��Һ��
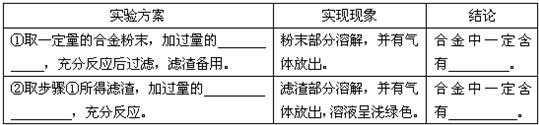
| ʵ�鷽�� | ʵ������ | ���� |
| 1ȡһ�����ĺϽ��ĩ���ӹ����� |
��ĩ�����ܽ⣬��������ų��� | �Ͻ���һ������ |
| ��ȡ����������������ӹ����� |
���������ܽ⣬��������ų�����Һ��dz��ɫ�� | �Ͻ���һ������ |
����˼��һ����˵�����ý�������������ᷴӦ���������ᡢ��ܷ�Ӧ��˵����������������ʣ�д������ϡ���ᷴӦ�Ļ�ѧ����ʽ
��֪ʶ���졿ͭ�����������������й㷺ʹ�õĽ�����
��1����ҵ����һ����̼�ͳ�������Ҫ�ɷ�����������ұ��������Ӧ�Ļ�ѧ����ʽΪ
��2��ͭ����Ҳ����������ɫͭ�⣬ͭ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ����ѧʽΪCu2��OH��2CO3�������Ԫ����
������������Ŀ������Ϣ������֤�Ͻ����Ƿ�����ʱ�����ǿ���������������������Һ��Ӧ����ƫ�����ƺ�������������������ͭ�ڼ���ʱ�����Ը��������������Ʋ���Ӧ�������ᷴӦ��ͭ���߶�����Ӧ�������ˣ�
����⣺���������IJ������ǿ���ȷ������2�����ܺ���ͭ�����ڼ���Ͻ�����ʱ���������úϽ��в�ͬ�Ľ����IJ�ͬ��ѧ���ʺ���������Ϣ�������˼����������Ƿ�����ʱ�����������ƺ�����Ӧ����������ȷ�����������Ƿ�����ʱ������ϡ���������飮�����Ƿ���ͭʱ������ͭ��ϡ����ܷ�Ӧ�����У�
�ʴ�Ϊ��
�����롿�²�2��ͭ
ʵ��̽��
����˼��2Al+6HCl=2AlCl3+3H2��
��֪ʶ���졿��1��3CO+Fe2O3
3CO2+2Fe ��2��4
�ʴ�Ϊ��
�����롿�²�2��ͭ
ʵ��̽��
| ʵ����� | ʵ������ | ʵ����� |
| ��30%NaOH��Һ | �� | |
| ��10%���� | ����ͭ |
��֪ʶ���졿��1��3CO+Fe2O3
| ||
������ԭ��ѧϰ�������ʱ�������ý�����Ӧ��������������Ϣ�У�����֪�������Ժ��������Ʒ�Ӧ��������Ҫ��ѧ��������֪ʶʱ������äĿ��������ѧ֪ʶ��Ҫ��������Ŀ���⾳�������Ŀ��

��ϰ��ϵ�д�
�����Ŀ

 ������Ϊ������Ľ������������绯ѧʷ���أ�����������1825��ű�Ӣ����ѧ�Ҵ�ά�Ƶã����죬���Ѿ����������������ÿһ�����䣮
������Ϊ������Ľ������������绯ѧʷ���أ�����������1825��ű�Ӣ����ѧ�Ҵ�ά�Ƶã����죬���Ѿ����������������ÿһ�����䣮