��Ŀ����
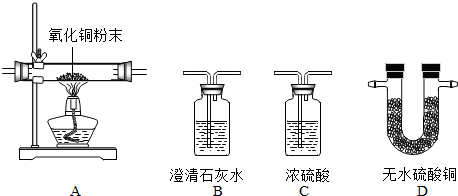
��2012?������һģ����1��ij��ѧ��ȤС��������ͼװ��̽����ȡ�����ԭ�������������ʣ����װ��ͼ���ش��������⣺
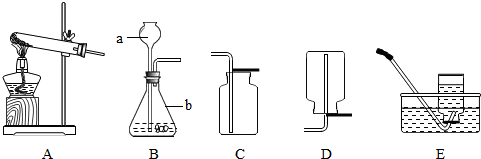
��д��ͼ�б�����������ƣ�a
����˫��ˮ�Ͷ���������ȡ����ʱ����ѡ�õķ���װ����
������ƿ����������˿��������ȼ��ʵ�飺��˿��������ȼ�յ�ʵ������
��2��ʵ�������д���ʯ��������ء�ϡ���ᡢϡ�������ɫʯ����Һ����ص���������Ʒ��С��ͬѧҪͨ��ʵ����֤������̼����ˮ��Ӧ�����ʣ�������ͼ�ش��������⣺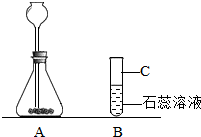
������A��B����ʵ��װ��ͼ����������
��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��B�й۲쵽��ʵ��������
����һ�����������C��������

��д��ͼ�б�����������ƣ�a
����©��
����©��
��b��ƿ
��ƿ
������˫��ˮ�Ͷ���������ȡ����ʱ����ѡ�õķ���װ����
B
B
������ţ�����ѡ��Cװ���ռ���������ԭ�����������ܶȱȿ�����
�������ܶȱȿ�����
��������ƿ�������ռ����ķ�����������ľ�����ڼ���ƿ�ڣ�����ȼ֤����
������ľ�����ڼ���ƿ�ڣ�����ȼ֤����
��������ƿ����������˿��������ȼ��ʵ�飺��˿��������ȼ�յ�ʵ������
����ȼ�գ��������䣬���ɺ�ɫ���壬�ų�����
����ȼ�գ��������䣬���ɺ�ɫ���壬�ų�����
����Ӧ�Ļ�ѧ����ʽ3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
����ʵ���м���ƿ��ը���ˣ�ԭ��
| ||
����ƿ��û�з�����ˮ���̻�ɳ
����ƿ��û�з�����ˮ���̻�ɳ
����2��ʵ�������д���ʯ��������ء�ϡ���ᡢϡ�������ɫʯ����Һ����ص���������Ʒ��С��ͬѧҪͨ��ʵ����֤������̼����ˮ��Ӧ�����ʣ�������ͼ�ش��������⣺

������A��B����ʵ��װ��ͼ����������
��A�з�����Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2 +CO2��+H2O
CaCO3+2HCl�TCaCl2 +CO2��+H2O
����B�й۲쵽��ʵ��������
��ɫʯ����Һ���ɫ�����ҵ��ܿ�������ð����
��ɫʯ����Һ���ɫ�����ҵ��ܿ�������ð����
������һ�����������C��������
���������Լ��ķ�Ӧ�������ڳ��»����ʱʹ��
���������Լ��ķ�Ӧ�������ڳ��»����ʱʹ��
����������1������Ϥ�����������˽����ǵ����ƣ�
�ڸ�����˫��ˮ�Ͷ���������ȡ�������ǹ�Һ�����ȷ�Ӧѡ����װ�ã������������ܶȷ�����ѡ��Cװ���ռ�������ԭ������������ȼ�Լ����Ƿ�������
����˿��������ȼ�յ�ʵ��������ȼ�գ��������䣬���ɺ�ɫ���壬�ų����������ݷ�Ӧ���Ӧ������������д�ɻ�ѧ��Ӧʽ��
��2����ע����ƿ�еĵ��ܲ��������Թ��ڵĵ���Ҫ����Һ��һ�£�
��ʵ������ȡ������̼�ô���ʯ��ϡ���ᷴӦ��
�۸���ʯ����Һ�����������
���Թ����������Լ��ķ�Ӧ�������ڳ��»����ʱʹ�ã�
�ڸ�����˫��ˮ�Ͷ���������ȡ�������ǹ�Һ�����ȷ�Ӧѡ����װ�ã������������ܶȷ�����ѡ��Cװ���ռ�������ԭ������������ȼ�Լ����Ƿ�������
����˿��������ȼ�յ�ʵ��������ȼ�գ��������䣬���ɺ�ɫ���壬�ų����������ݷ�Ӧ���Ӧ������������д�ɻ�ѧ��Ӧʽ��
��2����ע����ƿ�еĵ��ܲ��������Թ��ڵĵ���Ҫ����Һ��һ�£�
��ʵ������ȡ������̼�ô���ʯ��ϡ���ᷴӦ��
�۸���ʯ����Һ�����������
���Թ����������Լ��ķ�Ӧ�������ڳ��»����ʱʹ�ã�
����⣺��1����ͼ��a�dz���©����b����ƿ��
�ʴ�Ϊ������©������ƿ��
����˫��ˮ�Ͷ���������ȡ����ʱ���ǹ�Һ�����ȷ�Ӧ����ѡ�õķ���װ����B����Ϊ�������ܶȱȿ��������Կ�ѡ��Cװ���ռ���������������ȼ�����ԣ���������ʱ��������ľ�����ڼ���ƿ�ڣ�����ȼ֤������
�ʴ�Ϊ��B���������ܶȱȿ���������ľ�����ڼ���ƿ�ڣ�����ȼ֤������
����˿��������ȼ�յ�ʵ��������ȼ�գ��������䣬���ɺ�ɫ���壬�ų�����������������������ʵ���м���ƿ��ը������Ϊ����ƿ��û�з�����ˮ���̻�ɳ�����ɵĸ����������ը��ƿ�ף�
�ʴ�Ϊ������ȼ�գ��������䣬���ɺ�ɫ���壬�ų�������3Fe+2O2
Fe3O4������ƿ��û�з�����ˮ���̻�ɳ��
��2����ע����ƿ�еĵ��ܲ��������Թ��ڵĵ���Ҫ����Һ��һ�£���ͼ��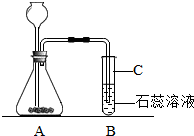
��ʵ������ȡ������̼�ô���ʯ��ϡ���ᷴӦ����ѧ��Ӧʽ�ǣ�CaCO3+2HCl�TCaCl2 +CO2��+H2O��
�ʴ�Ϊ��CaCO3+2HCl�TCaCl2 +CO2��+H2O��
��A�����ɵĶ�����̼��ˮ��Ӧ����̼�ᣬʯ����Һ�����죬����B�й۲쵽��ʵ����������ɫʯ����Һ���ɫ�����ҵ��ܿ�������ð������
�ʴ�Ϊ����ɫʯ����Һ���ɫ�����ҵ��ܿ�������ð������
���Թ����������Լ��ķ�Ӧ�������ڳ��»����ʱʹ�ã�
�ʴ�Ϊ�����������Լ��ķ�Ӧ�������ڳ��»����ʱʹ�ã�
�ʴ�Ϊ������©������ƿ��
����˫��ˮ�Ͷ���������ȡ����ʱ���ǹ�Һ�����ȷ�Ӧ����ѡ�õķ���װ����B����Ϊ�������ܶȱȿ��������Կ�ѡ��Cװ���ռ���������������ȼ�����ԣ���������ʱ��������ľ�����ڼ���ƿ�ڣ�����ȼ֤������
�ʴ�Ϊ��B���������ܶȱȿ���������ľ�����ڼ���ƿ�ڣ�����ȼ֤������
����˿��������ȼ�յ�ʵ��������ȼ�գ��������䣬���ɺ�ɫ���壬�ų�����������������������ʵ���м���ƿ��ը������Ϊ����ƿ��û�з�����ˮ���̻�ɳ�����ɵĸ����������ը��ƿ�ף�
�ʴ�Ϊ������ȼ�գ��������䣬���ɺ�ɫ���壬�ų�������3Fe+2O2
| ||
��2����ע����ƿ�еĵ��ܲ��������Թ��ڵĵ���Ҫ����Һ��һ�£���ͼ��

��ʵ������ȡ������̼�ô���ʯ��ϡ���ᷴӦ����ѧ��Ӧʽ�ǣ�CaCO3+2HCl�TCaCl2 +CO2��+H2O��
�ʴ�Ϊ��CaCO3+2HCl�TCaCl2 +CO2��+H2O��
��A�����ɵĶ�����̼��ˮ��Ӧ����̼�ᣬʯ����Һ�����죬����B�й۲쵽��ʵ����������ɫʯ����Һ���ɫ�����ҵ��ܿ�������ð������
�ʴ�Ϊ����ɫʯ����Һ���ɫ�����ҵ��ܿ�������ð������
���Թ����������Լ��ķ�Ӧ�������ڳ��»����ʱʹ�ã�
�ʴ�Ϊ�����������Լ��ķ�Ӧ�������ڳ��»����ʱʹ�ã�
���������⿼����������ȡ���ռ�����������˿��ȼ�ա�������̼����ȡ�����ʣ��ѶȲ������ص㣬����������

��ϰ��ϵ�д�
�����Ŀ