��Ŀ����
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6mLˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ���õ�����ͼ��ʾ�����߹�ϵ��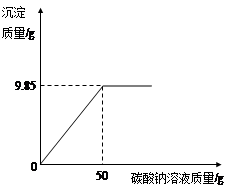
����Ա��С�����ʾ��
�ٷ�Ӧ�Ļ�ѧ����ʽ��BaCl2��Na2CO3��BaCO3����2NaCl
��ˮ���ܶȣ�1g/cm3
���Ʒ���Ȼ��Ƶ�����������
36%
����������������������ݴ����Ļ�ѧ����ʽ�����⣬����ͼ����֪���������ĵ�̼������Һ����Ϊ50gʱ����Ӧ��������ʱ���ɵ�̼�ᱵ��������Ϊ9.85g���ʿɸ��ݷ���ʽBaCl2��Na2CO3��BaCO3����2NaCl��̼�ᱵ��BaCl2��������ϵ�������BaCl2�������������������Ʒ�е��Ȼ��������������������Ʒ���Ȼ��Ƶ���������
�⣺����Ʒ��BaCl2������Ϊx
Na2CO3������BaCl2��BaCO3����2NaCl
106����������208
50g��10.6������x ��x��10.4g
��x��10.4g
��Ʒ��NaCl����������Ϊ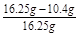 ��100%��36%
��100%��36%
�𣺲�Ʒ��NaCl����������Ϊ36%��
���㣺���ݻ�ѧ����ʽ����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
ijѧ����������ͼ��ʾ��ʵ�顣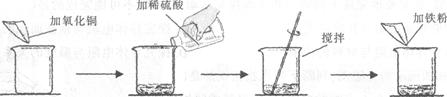
| | ��һ�� | �ڶ��� |
| ��������ͭ������ | m | m |
| ����ϡ��������� | 50g | 100g |
| �������۵����� | 5��6g | 5��6g |
| ʵ������ | ��ɫ������Ϻ�ɫ���� | �Ϻ�ɫ���� |
���ڶ�����������ǡ����ȫ��Ӧ(��Һ��ʧ���Բ���)����ش��������⣺
��1��д��ʵ���з�����Ӧ�Ļ�ѧ����ʽ
��2����һ��ʵ���Ĺ������ʵĻ�ѧʽΪ
��3��������֪�����г����ڶ���ʵ�����ɹ������ʵ�����(x)�ı���ʽ
��4��ʵ���м�������ͭ������(m)Ϊ ��
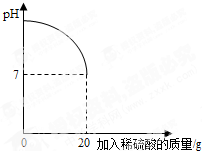
��5������ڶ��η�Ӧ�����Һ�м���92��8gˮ�������ò�������Һ�����ʵ���������Ϊ ��
��6������������������Ϊ49����������Һ����ʵ���������ϡ���ᣬ����Ҫ��ˮ������
Ϊ ��
 2CO2+3H2O������100g��������Ϊ92%���Ҵ���Һ�ڿ�������ȫȼ�ղ���������̼������Ϊ���ٿˣ�
2CO2+3H2O������100g��������Ϊ92%���Ҵ���Һ�ڿ�������ȫȼ�ղ���������̼������Ϊ���ٿˣ�  2H2��+ O2 ����
2H2��+ O2 ����