��Ŀ����
����Ŀ����18����ʵ���Ҵ���һ�����ڵĻ�ѧҩƷ����ȤС��ͬѧ������һѢ�Ѿ���������������Ʒչ��̽����������롣
[̽���һ]֤�����ʵ���Ʒ��Ȼ���������ƴ���
����ժҪ��̼���Ƶ�ˮ��Һ�ʼ��ԣ��Ȼ��ơ��Ȼ��Ƶ�ˮ��Һ�����ԡ�
��1���������Ʊ�¶�ڿ����з������ʷ�Ӧ�Ļ�ѧ����ʽΪ ��
ͬѧ�ǰ���������ʾ�IJ������ʵ�飺
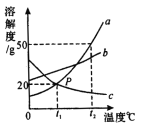
��2��������м���������Ȼ�����Һ��Ŀ���� ������A�ض����е������� ��д��ѧʽ����
��3��������ʵ�顱��֤�������ƵĴ��ڣ��������ʵ�鲽��Ͳ��������� ��
[̽�����]�ⶨ��Ʒ��̼���Ƶ���������
ȡ5.3g��Ʒ��������ϡ���ᷴӦ���������ɶ�����̼��������Ӷ������̼���Ƶ�����������ʵ��װ����ͼ���������������ص�Ӱ�죬װ�õ���������������
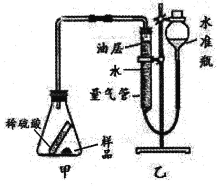
��4����б��ƿʹ��Ʒ��ϡ�����ֽӴ���д����װ���з�����Ӧ��һ����ѧ����ʽ�� ��
��5����������ˮ����Ҫ��һ��ֲ���ͣ�������̼�����ڸ��Ͳ�����Ŀ���� ��
��6��ʵ���õIJ����������±���ʾ��
��Ӧǰ | ��Ӧ�� | |
��������Һ���Ӧ�Ŀ̶� | 42mL | 262mL |
���ݱ��е�ʵ�����ݼ��㣬��Ӧ���ɵĶ�����̼���Ϊ mL����֪��ʵ�������£�������̼���ܶ�Ϊ2g��L��1�������ɶ�����̼������Ϊ g��
��7��ͨ�����㣬��Ʒ��̼���Ƶ���������Ϊ ��
���𰸡���1��2NaOH + CO2 === Na2CO3 + H2O ��2��������Һ�е�̼��������� CaCO3
��3��ȡ������ҺB���Թ�����������ɫ�ķ�̪��Һ����Һ�ʺ�ɫ��
��4��Na2CO3+H2SO4 === Na2SO4+H2O+CO2������2NaOH+H2SO4 === Na2SO4+2H2O��
��5����ֹ������̼����ˮ���ʵ����� ��6��220 0.44 ��7��20%
��������
�����������1���������Ʊ�¶�ڿ����з������ʣ���������еĶ�����̼������Ӧ����ѧ����ʽΪ��2NaOH + CO2 === Na2CO3 + H2O
��2��Ϊ�˲�Ӱ�����ʵ����������ƵĴ�������жϣ���������м���������Ȼ�����Һ��Ŀ����������Һ�е�̼������ӵ�����Ӧ�ķ�ӦΪ��CaCl2+Na2CO3==CaCO3��+2NaCl��������A�ض����е�������CaCO3
��3��������ʵ�顱��֤�������ƵĴ��ڣ�ʵ�鲽��Ͳ���������ȡ������ҺB���Թ��У�������ɫ�ķ�̪��Һ����Һ�ʺ�ɫ��
��4������ҩƷ�����������ƺ�̼���ƣ�����б��ƿʹ��Ʒ��ϡ�����ֽӴ���������Ӧ��һ����ѧ����ʽ��Na2CO3+H2SO4 === Na2SO4+H2O+CO2������2NaOH+H2SO4 === Na2SO4+2H2O��
��5�����ڶ�����̼������ˮ������������ˮ����Ҫ��һ��ֲ���ͣ�������̼�����ڸ��Ͳ�����Ŀ���ǣ���ֹ������̼����ˮ���ʵ�����
��6�����ݱ��е�ʵ�����ݼ��㣬��Ӧ���ɵĶ�����̼���=262 mL- 42 mL =220 mL����������=�ܶȡ�����ļ���ʽ����֪��ʵ�������£�������̼���ܶ�Ϊ2g��L��1�������ɶ�����̼������=220��10-3L��2g��L��1 = 0.44g
��7�����ݻ�ѧ����ʽ��Na2CO3+H2SO4 === Na2SO4+H2O+CO2����CO2��Na2CO3��������ϵ�������Na2CO3����������һ��������Ʒ��̼���Ƶ���������
�⣺��Na2CO3������Ϊx
Na2CO3+H2SO4 === Na2SO4+H2O+CO2��
106 44
x 0.44g
106:44=x��0.44g
x=1.06g
��Ʒ��̼���Ƶ���������=1.06g/5.3g��100%=20%

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�