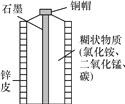
��Ŀ����
����Ŀ������Ӧ�Ļ�ѧʽ��Ԫ�ط��ű�ʾ��
��1������_____��3����ԭ��_____��+3�۵���Ԫ��_____������_____ 5���������_____��4��������_____�����û�ѧʽ��ʾ���л�ѧ��Ӧ��
��2��þ�ڿ�����ȼ�գ�_____
��3��ͨ��ֽ�ˮ��_____
��4����˿�������о���ȼ�գ��������䣬���ɺ�ɫ���壮_____
��5������������5�����Ļ����������N2H4����ԭ�ϣ���������������N2O4����ȼ��ȼ�պ���������֮һ�ǵ�������һ�������ﳣ����ΪҺ̬��_____��
��6������ˮ����������������������ˮ�ʣ���Ϊ������ˮ��Ӧ���������ᣨHCl���ʹ����ᣨHClO�������������ɱ���������ã�_____������������������Һ��Ӧ���Ƶ�Ư�ۣ�����������Ȼ��ƵĻ�����_____��
���𰸡�H2 3Na ![]() S 5H2S 4Al3+ 2Mg+O2
S 5H2S 4Al3+ 2Mg+O2![]() 2MgO 2H2O
2MgO 2H2O ![]() 2H2��+O2�� 3Fe+2O2
2H2��+O2�� 3Fe+2O2![]() Fe3 O4 2N2H4+N2O4
Fe3 O4 2N2H4+N2O4![]() 3N2��+4H2O Cl2+H2O=HCl+HClO 2Cl2+2Ca��OH��2 =CaCl2+Ca��ClO��2+2H2O
3N2��+4H2O Cl2+H2O=HCl+HClO 2Cl2+2Ca��OH��2 =CaCl2+Ca��ClO��2+2H2O
��������
������Ҫ���컯ѧ�����Լ��仯ѧʽ����ȷ��д
��1�������Ļ�ѧʽΪ��H2��3����ԭ�ӿ��Ա�ʾΪ3Na��+3�۵���Ԫ�ؿɱ�ʾΪ![]() ������ΪS��
������ΪS��
5��������ӿ��Ա�ʾΪ��5H2S��4�������ӿɱ�ʾΪ4Al3+
��2��þ�ڿ�����ȼ����������þ����Ӧ�Ļ�ѧ����ʽΪ��4P+5O2![]() 2P2O5��
2P2O5��
��3��ˮͨ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O![]() 2H2��+O2����
2H2��+O2����
��4������������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ3Fe+2O2![]() Fe3O4��
Fe3O4��
��5��������N2H4����ԭ�ϣ���������������N204����ȼ��ȼ�պ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ2N2H4+N2O4![]() 3N2��+4H2O
3N2��+4H2O
��6��������ˮ��Ӧ���������ᣨHCl���ʹ����ᣨHClO������ѧ����ʽΪCl2+H2O=HCl+HClO
����������������Һ��Ӧ���Ƶ�Ư�ۣ�����������Ȼ��ƵĻ�����ѧ����ʽΪ2Cl2+2Ca��OH��2 =CaCl2+Ca��ClO��2+2H2O��

����Ŀ���ס��ҡ����������ʵ�ת����ϵ����ͼ��ʾ����������ʾ��Ӧһ��ʵ�֣��������ʺͷ�Ӧ��������ȥ��������ѡ���ʵ��ͼʾת�����ǣ� ��
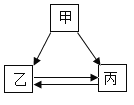
ѡ�� | �� | �� | �� |
A | H2SO4 | H2 | H2O |
B | C | CO | CO2 |
C | CuSO4 | Cu(OH)2 | CuCl2 |
D | NaOH | NaCl | NaNO3 |
A.AB.BC.CD.D