��Ŀ����
����Ŀ����ѧС��ͬѧ������������������м�����һЩ��ɫ��ĩ��ʹ������������ͬѧ�Ƕ�ɫ��ĩ�ijɷֽ���̽����
���������ϣ��پ����飬�г���������3����Ҫ��Ʒ��
��Ʒ���� | С�մ� | ʳ���� | ��ϼ������ɼ� |
��Ҫ�ɷ� | ̼������ | ̼����� | ̼�����ƺ�̼����� |
��2NaHCO3![]() Na2CO3+H2O+CO2����NH4HCO3
Na2CO3+H2O+CO2����NH4HCO3![]() NH3��+H2O+CO2����2NH3+H2SO4=��NH4��2SO4
NH3��+H2O+CO2����2NH3+H2SO4=��NH4��2SO4
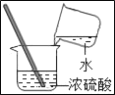
��ʵ������ͬѧ��ѡ������ʵ��װ�ã�����Ͻ���ʵ�飺
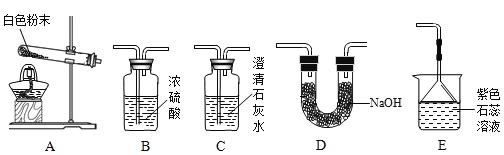
��ʵ���¼��
ʵ����� | ʵ��װ�� | ʵ������ | ʵ����ۼ����� |
ʵ��1 | ѡ��A��B��C | C�г���ʯ��ˮ����� | ��CO2���ɣ�C�з�Ӧ�Ļ�ѧ����ʽ��_____ |
ʵ��2 | ѡ��A��_____��E | E����ɫʯ����Һ��_____ɫ | ��NH3���� |
��ʵ�������
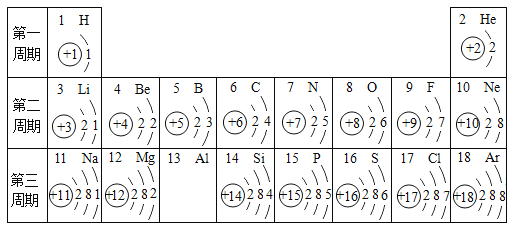
��������ʵ�飬��ͬѧ��Ϊ�÷�ĩ����Ҫ�ɷ�Ϊʳ���ۣ���ͬѧ��Ϊ����ʵ�鲻��ȷ���÷�ĩΪʳ���ۡ�Ϊ��һ��ȷ������ɼ�������ͬѧ����������¶���ʵ�飺
ʵ����� | ʵ��Ŀ�� | ʵ��װ�� | ���ݼ�¼ | ʵ����� |
ʵ��3 | �ⶨ����CO2������ | ѡ��A��B��D | װ��D����ag | �÷�ĩΪ����ϼ������ɼ��� |
ʵ��4 | �ⶨ����NH3������ | ѡ��A��D��B | װ��B����1.7g |
��1��ʵ��3��װ��B��������_____��
��2��ʵ��3�в��װ��D����ag����a>_____g��
��3�������aΪ92.4���ü������ɼ���NaHCO3��NH4HCO3��������Ϊ_____
���𰸡�Ca��OH��2+CO2�TCaCO3��+H2O D �� ����ˮ�����Ͱ��� 4.4 3360��79
��������
ʵ���¼��
ѡ��A��B��C��C�г���ʯ��ˮ����ǣ��Ƕ�����̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ����ѧ����ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��ѡ��A��D��E��D�����ն�����̼��ˮ�����ģ�E�Ǽ��鰱���ģ���������ˮ�ǰ�ˮ����ˮ��ʹ��ɫʯ����Һ����ɫ��
ʵ��
��1��ʵ��3��װ��B�������ǣ�����ˮ�����Ͱ�����
��2��װ��D���ص������Ƕ�����̼�����������װ��B����1.7g����Ϊ̼��������ȷֽ�����һ�ݰ�����һ�ݶ�����̼����ô���ɶ�����̼��������4.4g���μӷ�Ӧ��̼����淋�����Ϊ7.9g������Ϊ̼�����Ʒֽ�Ҳ���ɶ�����̼�����a����4.4g��
��3�������aΪ92.4g����ô̼�����Ʒֽ����ɵĶ�����̼������=92.4g-4.4g=88g������88g������̼��������̼�����Ƶ�������Ȼ����NaHCO3��NH4HCO3�������ȣ�������88g������̼��Ҫ̼�����Ƶ�����Ϊx��

![]() x=336g�����NaHCO3��NH4HCO3��������=336g��7.9g=3360��79��
x=336g�����NaHCO3��NH4HCO3��������=336g��7.9g=3360��79��

����Ŀ��������Ҧ��С����ѪҺ��ijЩԪ�ؼ�����ı��浥(����)�����ݴ˷ݱ��浥������Լ���ѧ֪ʶ�ش��������⣺��������Ҧ�� �Ա�Ů ���䣺5�ꣻ�걾���ࣺѪ�����ţ�YS0130�������ڣ�2009-9-10��
��� | �����Ŀ | ��� | �ο�ֵ |
1 | п | 7.7 | 11��22����mol��L��1 |
2 | ͭ | 16.9 | 14��29����mol��L��1 |
3 | �� | 17.8 | 9.0��21.5��mol��L��1 |
4 | �� | 2.0 | 2.2��2.7��mol��L��1 |
5 | þ | 0.84 | 0.8��1.2��mol��L��1 |
6 | Ǧ | 0.28 | 0��0.48��mol��L��1 |
��1��Ҧ��ȱ���ij���Ԫ����______(��Ԫ�ط���)��Ҧ��ȱ����Ԫ�ػ�_______�������Ŀ�������к�Ԫ�ص���_____(��Ԫ������)��
��2�����ݼ������ҽ��������ÿ�첹��10 mg��п��Ҧ�صİְָ�����������IJ�п����
��ҩƷ���ƣ���������пƬ
����״��Ƭ����ζ����ɬ���б���ζ��
��ҩƷ��ɣ�ÿƬ����������п��C12H22O14Zn��35���ˣ���ͨ������˵��Ҧ��ÿ��һ��Ӧ�ó�____________Ƭ��
��3��Ҧ��������Ϊ��������пƬ��Ӫ����������Լ�Ƭû��ϵ������������һ�۵㣬��˵������________________��
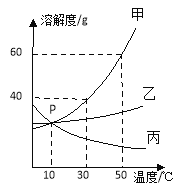
����Ŀ��ͨ��ѧϰ�����ǿ��Դӻ�ѧ�ĽǶȿ����⡣���������ڿ�ݷ��������ǧ���������ͼʾ�ش�
��1���������������ڽ�����Ͻ����_____��ѡ��һ�����ʱ�ţ��������л��ϳɲ��ϵ���_____��ѡ��һ�����ʱ�ţ���
��2�������ֱ�����������_____���ϣ�������ԡ����ȹ��ԡ�����
��3�������ѳ�Ϊ�ڶ��ͥ�������Ʒ�������Ƕ�����һЩӪ���ɷֵ�ƽ������������������±��ش�
�ɷ� | ˮ | ������ | ֬�� | ���� | �� | �� | �� | ά����A |
��������/% | 96.0 | 1.8 | 0.7 | 1.1 | 0.01 | 0.03 | 0.0005 | 0.015 |
�ٶ����к��е���Ԫ����_____��
��������ȱ��_____Ԫ���������Ͳ���
���ڶ���������Ӫ�������ܹ�������������Ӫ������_____��
����������ÿ��ʳ��90g�����������뵰���ʵ�����Ϊ_____g��