��Ŀ����
��6�֣�Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⡣
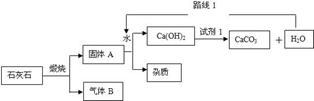
��1����ȥ�����л��е�����������̼���壺
�ٳ���ʱ�������������������� ���a����b�����˽���ϴ��ƿ��
��A��B�ж�ʢ�������ij���ʯ��ˮ��B�г���ʯ��ˮ�������� ��������
����ʱ��˵��CO2�ѳ�����
��2���ø�װ����֤������̼�Ƿ���ˮ��Ӧ���ṩ��ҩƷ��
����ɫʯ����Һ ����ɫʯ����ҺȾ�ɵĸ���ֽ����
A��B��Ӧ����ʢ�� ����ҩƷ��ţ��������֤��������̼��ˮ�����˻�ѧ��Ӧ���÷�Ӧ��ѧ����ʽΪ ��

��1����ȥ�����л��е�����������̼���壺
�ٳ���ʱ�������������������� ���a����b�����˽���ϴ��ƿ��
��A��B�ж�ʢ�������ij���ʯ��ˮ��B�г���ʯ��ˮ�������� ��������
����ʱ��˵��CO2�ѳ�����
��2���ø�װ����֤������̼�Ƿ���ˮ��Ӧ���ṩ��ҩƷ��
����ɫʯ����Һ ����ɫʯ����ҺȾ�ɵĸ���ֽ����
A��B��Ӧ����ʢ�� ����ҩƷ��ţ��������֤��������̼��ˮ�����˻�ѧ��Ӧ���÷�Ӧ��ѧ����ʽΪ ��
��1����a������֤������̼�Ƿ�����B�г���ʯ��ˮ����������ʱ���������ɣ���
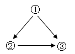
��2���ڢ٣�CO2 +H2O = H2CO3
��2���ڢ٣�CO2 +H2O = H2CO3
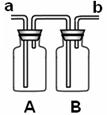
��1����Ϊ�����Ͷ�����̼���ܶȶ��ȿ�������a�˽���ϴ��ƿ��������̼�����ü�Һ���գ���ʯ��ˮ���飬A��B�ж�ʢ�������ij���ʯ��ˮ��A�����ն�����̼��B�Ǽ���CO2�Ƿ��ѳ�����
��2��Ҫ��֤������̼�Ƿ���ˮ��Ӧ��A��Ӧ�Ÿ������ɫʯ����ֽ���ޱ仯��B�з���ɫʯ����Һ����ɫ���ɫ��������̼��ˮ��Ӧ������̼�ᣮ
��2��Ҫ��֤������̼�Ƿ���ˮ��Ӧ��A��Ӧ�Ÿ������ɫʯ����ֽ���ޱ仯��B�з���ɫʯ����Һ����ɫ���ɫ��������̼��ˮ��Ӧ������̼�ᣮ

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ