��Ŀ����
����Ŀ����ȤС���������з����ⶨCO2�����������������ʯ��ʯ��̼��ƵĴ��ȣ���̼����⣬�������ʲ������ᷴӦ����
����һ���ⶨCO2������
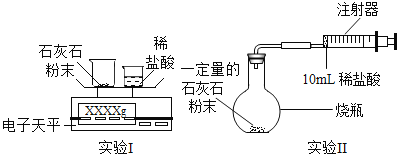
��1��������ʵ���н�ʯ��ʯ��ĥ�ɷ۵�Ŀ����_____��
��2���ڸ�ʵ������У��ò��������Ͻ����������_____��
a����ʹʯ��ʯ�������ַ�Ӧ b�������ڶ�����̼��ɢ��
��3��ʵ��I�У���С�ձ��е�ϡ����ּ��μ��뵽���ձ��У������Ͻ��裬�ж�ʯ��ʯ��̼�����ȫ��Ӧ�IJ�����ʵ�������ǣ����һ�μ���ϡ���ᣬ_____��
���������ⶨCO2�����
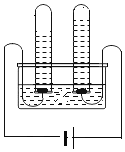
��4��ʵ��II�У����Ӻ�װ�ã���_____����������ƣ���Ȼ��װ��0.5gʯ��ʯ��Ʒ�����10mlϡ�������������ƿ�С�
��5��ʵ��II��ʵ���¼���£�������������ͬ�¶ȡ���ͬѹǿ�����²ⶨ����
ʱ��/min | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
ע��������/ml | 60.0 | 85.0 | 88.0 | 89.0 | 91.5 | 92.5 | 98.9 | 99.6 | 99.6 | 99.6 |
��������ʵ����̺������ۺϷ�������������CO2�������_____ml��
��6�����ݼ��㣺��֪���³�ѹ��CO2���ܶ���1.96g/L����÷���������CO2������Ϊ_____g����ȷ��0.001g����ʯ��ʯ�Ĵ���Ϊ_____��
��7��ʵ�鷴˼����ͬѧ��Ϊʵ��������ʹ��ȷ���ⶨ���Ҳ��ȷ��ԭ����_____��
���𰸡�����Ӵ������ʹ���ַ�Ӧ�� ab �����ݲ��� ���װ�õ������� 89.6ml 0.176g 80% ������̼�ݳ�ʱ������ˮ����
��������
�⣺��1����ĥ�ɷ������˷�Ӧ��ĽӴ�������ӿ��˷�Ӧ�����ʣ����Խ�ʯ��ʯ��ĥ�ɷ۵�Ŀ���ǣ�����������ĽӴ������ʹ̼�����ȫ��Ӧ��
��2����������Ƿ�Ӧ���ٷ�����Ҳ��С�˶�����̼���ܽ����������ԣ����Ͻ��裬��ʹ̼����������ַ�Ӧ��ͬʱ�����ڶ�����̼��ɢ�ݣ�
��3��ʯ��ʯ�����ᷴӦ�ų�������̼���壬���ԣ��ж�ʯ��ʯ��CaCO3��ȫ��Ӧ��ʵ�����������һ�μ���ϡ���ᣬ��û�����ݲ�����
��4����ȡ����Ҫ�ȼ��װ�õ������ԣ�Ȼ��װ��ҩƷ�����10mlϡ�������������ƿ�У�
��5���ӵ�8���Ӻ������������ٱ仯����ԭϡ����ռ��10mL������ϡ�������Ȼ��ָ���ԭѹǿ�������Ե�1��������CO2�������50mL����2������75mL����������CO2�������89.6mL��
��6������CO2������=89.6mL![]() 1.96g/L=0.176g����μӷ�Ӧ̼��Ƶ�����Ϊx��
1.96g/L=0.176g����μӷ�Ӧ̼��Ƶ�����Ϊx��
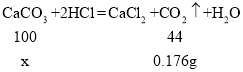
![]()
x=0.4g��
ʯ��ʯ�Ĵ���=![]() ��
��
��7��ʵ���Ϊ����װ�ã����ɵĶ�����̼�ܵ�������ͬʱ���������ˮ��

����Ŀ����ѧС������ˮ���ռ��˺������壬ͨ����ʵ��1���Ƚ���������ͺ�������ijɷֲ��졣
��ʵ��1��
��� | ʵ��Ŀ�� | ʵ����� | ʵ������ |
�� | �ȽϿ����ͺ��������ж�����̼�ĺ��� | ����������е�����������ʯ��ˮ���� | _____ |
�� | �ȽϿ����ͺ��������������ĺ��� | �ֱ�ȼ�ŵ�ľ����������ͺ��������� | �����е�ľ�������Ա仯�����������е�ľ��Ϩ�� |
�� | _____ | ȡ������ﲣ��Ƭ����������һ����� | �����IJ���Ƭ�ϳ�����ɫҺ�Σ���һ�������Ա仯 |
��1��ʵ��ٵ�����Ϊ_____��֤�����������з�����_____������������ѧ�����仯��
��2��ʵ��۵�Ŀ����_____��
��3��С��ָ��ʵ��ٲ��ܱȽϿ����ͺ���������CO2�ĺ��������貹���ʵ�����Ϊ_____��С���Ԣ���ȼ��ľ��Ϩ���ԭ��������룬����ƣ�ʵ��2��������֤��
����������裩
����1��ľ��Ϩ������Ϊ������������������CO2�ĺ����ߡ�
����2��ľ��Ϩ������Ϊ������������������O2�ĺ����͡�
��ʵ��2��
��� | �� | �� | �� | �� |
ʵ����� |
���� |
O2��CO2����� |
O2��N2����� |
O2��N2����� |
ʵ������ | ȼ�ŵ�ľ�������Ա仯 | ȼ�ŵ�ľ�������Ա仯 | ȼ�ŵ�ľ��Ϩ�� | ȼ�ŵ�ľ��Ϩ�� |
��4����ʵ��ٺ͢ڿɵó�����1_____���������������������
��5����ʵ��2���У���֤������2������ʵ�������_____��
��ʵ�鷴˼��
��6����������ʵ�飬����˵����ȷ����_____������ĸ��ţ���
A ��������ͺ���������O2��CO2�ĺ�����ͬ
B ��ȼ��ľ��������������У�ľ��Ϩ��˵������������û��O2
C ��ʵ��1���Ģ��У�ȼ��ľ��Ϩ���ԭ������ˮ�������������й�
D ����ȼ��ľ�����������Ϊ1:1��O2��CO2��������У���۲쵽�����Ա仯