��Ŀ����
����Ŀ����t1��ʱ����������������غ��Ȼ��طֱ���뵽��ʢ��100gˮ�������ձ��У���ֽ��貢�ָ���ԭ�¶Ⱥ�������ͼ1��ʾ��ͼ2������غ��Ȼ��ص��ܽ������
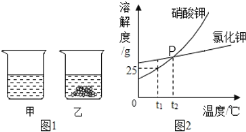
��1��ͼ2��P��ĺ���_____
��2���ձ�������Һ�Ƿ�ﵽ����״̬��_____���������������������ж������������ܽ��������_____
��3��Ҫ�ᴿ��������KCl��KNO3���壬�ɲ��õķ�����_____
��4������ͼ1�ձ��ڵ����ʣ�������ˮ������������˵����ȷ����_____
A �ձ��������ʵ��������������ձ��������ʵ���������
B ����һ������ˮ�����ձ��ж�һ���й�������
C �¶����ߵ�t2�����ձ����й���һ��ȫ�ܽ⣬����Ϊ��������Һ
D �������ձ��е���Һ��ϣ���ֽ�����ձ���һ������ʣ�����
E t1��ʱ�������Ƶ���ͬ�����������������Һ���Ȼ�����Һ
���𰸡�t2��ʱ��KNO3��KCl���ܽ����� ���ж� KCl�����Ȼ��أ� �ܽ⡢����Ũ������ȴ�ᾧ��ϴ�ӡ����ˡ����� AC
��������
��1��ͨ�������ܽ�����߿�֪��ͼ2��P��ĺ����ǣ�t2��ʱ��KNO3��KCl���ܽ����ȣ�
��2��t1��ʱ���Ȼ��ص��ܽ�ȴ�������ص��ܽ�ȣ������ձ����е���Һ�ײ�û�й���ʣ�࣬���Ը���Һ�Ƿ�ﵽ����״̬���жϣ������ܽ���������Ȼ��أ�
��3������ص��ܽ�����¶ȱ仯Ӱ��ϴ�����Ҫ�ᴿ��������KCl��KNO3���壬�ɲ��õķ������ܽ⡢����Ũ������ȴ�ᾧ��ϴ�ӡ����ˡ����
��4��A���ձ��ס����е��ܼ�������ȣ��ձ����еĹ�����ȫ�ܽ⣬�����ձ��������ʵ��������������ձ��������ʵ���������������ȷ��
B������һ������ˮ�����ձ��в�һ���й����������ʴ���
C���¶����ߵ�t2�棬����ء��Ȼ��ص��ܽ����ȣ������ձ����й���һ��ȫ�ܽ⣬����Ϊ��������Һ���ʴ���
D���������ձ��е���Һ��ϣ���ֽ�����ܼ���������һ���������ձ���һ��û��ʣ����壬�ʴ���
E��t1��ʱ���������ɱ仯��Һ���Ȼ�����ɲ�������Һ�������Ƶ���ͬ�����������������Һ���Ȼ�����Һ���ʴ���

����Ŀ����������ĸ��ʾ��8��������H��C��O��Na��Cl��Ca��Mn�еļ���Ԫ����ɣ������dz��л�ѧ���������ʣ�
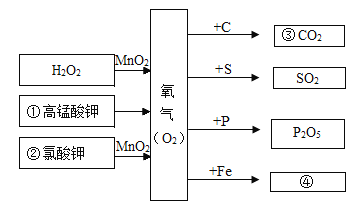
��1��A�Ǵ���ʯ����Ҫ�ɷ֣���B��Һ�������ݣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��2��X��Y��Ӧ����A��Z������Y��Z����������X�к�3��Ԫ�أ�Z�Ļ�ѧʽΪ_____��X�Ļ�ѧʽΪ_____��
��3����D����μ���C��������һ��������������壬C��Z������ͬ��Ԫ�أ��÷�Ӧ�Ļ�ѧ����ʽΪ_____��
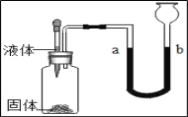
��4����������������ѡ2��Ϊһ�飬����ͼ��ʾװ�ý���ʵ�飬����ͷ�ι��е�Һ�����ƿ�У�a��ˮ�潵�ͣ�b��ˮ�����ߣ�д������Ҫ���4�����ʣ���������ʾ����д���ʵĻ�ѧʽ��
| �� | �� | �� | �� |
Һ�� | ______ | ______ | ______ | ______ |
���� | ______ | ______ | ______ | ______ |
����Ŀ�������NaCl��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
��1����Ҫ�Ƚ�KNO3�� NaCl��ˮ�е��ܽ���������Ҫ���Ƶı�����ˮ��������______��
��2��60��ʱ����100 gˮ�м���100 g KNO3���壬��ֽ����õ�______����������������������������Һ����ʱ����Һ�����ʵ���������Ϊ______�������¶���ȴ��20�����ձ����������������Ϊ______g����ʱ��Һ��������������Ϊ______����ȷ��0.01%����
��3����10��ʱ��NaCl������Һ��������������______������������������������С������KNO3������Һ������������������50��ʱ��12gˮ��������ܽ�______ gNaCl��