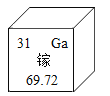
��Ŀ����
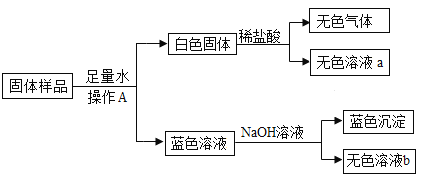
����Ŀ����֪A~G�������ʶ��dz��л�ѧ�α��г��ֹ��Ļ��������F�dz��õĽ������ϣ�HΪ�����ĵ��ʣ�������֮���������ת����ϵ����Ӧ��������ͼ��ʾ����

��1��д�����ʵĻ�ѧʽ��B_________��E_________��F_________��D_________����ɫ��Һ�е����ʰ���_______________��
��2��д����ѧ����ʽ��������������Ӧ���ͣ�
��__________________________________________������______________��Ӧ��
��__________________________________________������______________��Ӧ��
��__________________________________________������______________��Ӧ��
��3����Ӧ���ǷֽⷴӦ������A�а�����Ԫ����__________________��
���𰸡�CuO Ca(OH)2 CaCO3 CO2 CuSO4 ��H2SO4 H2SO4+ CuO=CuSO4+ H2O ���ֽ� Fe��CuSO4��Cu��FeSO4��Fe+2HCl =FeCl2��H2�� �û� CaCO3 ![]() CaO��CO2�� �ֽ� Cu��C��H��O
CaO��CO2�� �ֽ� Cu��C��H��O
��������
����F�dz��õĽ������ϣ�����FΪ̼��ƣ�̼��Ƹ������������ơ�������̼����GDӦ��Ϊ������̼�������ƣ�����Eͨ�������̼����F̼��ƣ��Ƴ�E���������ƣ�C��ˮ��C��G��Ӧ�����������ƣ�����G�������ƣ�D�Ƕ�����̼��
B�����ϡ���ᷴӦ���õ���ɫ��Һ��������ɫ��Һ��ͭ���ӵ���Һ��������ɫ��Һ������ͭ��Һ�����滹�й�����ϡ���
H�ǵ��ʲ�������ɫ��Һ��Ӧ������dz��ɫ��Һ����ɫ���塢��ɫ���壬����H������dz��ɫ��Һ��������������ɫ��������������ɫ������ͭ��
����B��������ᷴӦ��������ͭ����B��һ���ֽⷴӦ�õ�����������֮һ����һ��Ϊ��ʽ̼��ͭ�ֽ�Ľ��������B������ͭ��
��ΪA������������ͭ��ˮ��������̼������A�Ǽ�ʽ̼��ͭ��
�ʴ�Ϊ��
��1��CuO��Ca��OH��2��CaCO3�� CO2��CuSO4��H2SO4
��2����H2SO4+CuO�TCuSO4+H2O�� ���ֽ⣻
��Fe+CuSO4=Cu+FeSO4��Fe+H2SO4=FeSO4+H2�����û���
��CaCO3 ![]() CaO+CO2�����ֽ⣻
CaO+CO2�����ֽ⣻
��3��Cu��C��H��O��

 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ����СӢ�ҵIJֿ���ѷ���һ��������һһ̼�����( NH4HCO3).����һ�����죬СӢ�������ֻ��������еĴ̼�����ζ��ø�Ũ���ˣ���Щ���ʴ���̼����隣����ˣ���鷢�ֱ��ٵĻ��ʰ�װ��û���ܷ⣬����Ҳû�������ڵ��ϣ���û���˽����ֿ��ʹ�á�
Ϊ��̽����Щ���ʼ��ٵ�ԭ��СӢ��ʵ����ȡ��һЩ̼����立�ĩ�������������м��ȣ���һ����۲쵽��ĩ��ȫ��ʧ��ͬʱҲ�ŵ������ִ̼�����ζ.��ĩΪʲô����ʧ��?
(1)��������⣩̼����立�ĩ��ʧ��ԭ����ʲô?
(2)�����룩��̼����立�ĩ�ڲ����Ȼ�����������ɹ�̬���������̬����̼������ڲ����Ȼ���������·����ֽⷴӦ�����ܲ����������а�����һЩ�����
(3)���������ϣ���̼��������ڰ��ʣ����������������ʣ�˵����������__________ (�����)���������ڰ���(��ѧʽNH3)��������Ĵ̼�����ζ����������ˮ����ˮ��Һ�Ǽ��ԣ�������İ�������ʹ����ĺ�ɫʯ���Լ���������NO2Ϊ����ɫ���塣NOΪ��ɫ���壬�ڿ�����������Ӧ��2NO+O2=2NO2
(4)��ʵ���������������ۣ�
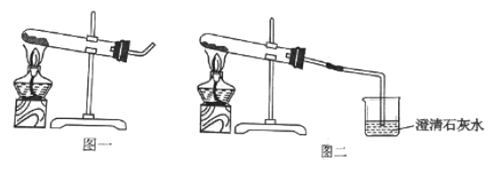
ʵ����� | ʵ������ | ʵ����� |
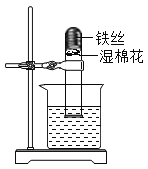
��ȡ����̼��������Թ��м��ȣ���ͼһ��ʾ��������ĺ�ɫʯ����ֽ�ӽ����ܿ� | ����ǿ�ҵĴ̼�����ζ���Թܱ�������ɫҺ������ֽ��������δ������ɫ���� | �ֽ��������______��û��__________ |
�ڰ���ͼ����ʾװ�ü���ʵ�飬ֱ����Ӧ��ȫ | ����ʯ��ˮ����� | �ֽ��������__________ |
(5)��Ӧ�ã������ð����Ļ�ѧ���ʣ���д��ʵ���Ҽ��鰱���ķ���(д��ʵ�������������)��________________________________________��
������������̼����炙��ʣ���Ӧ����α���? ______________________________��