��Ŀ����
����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ��������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������������ʾ����֮���ܹ�������ѧ��Ӧ��

�밴Ҫ��ش��������⣮
(1)�����������A����������________����B����������________(�������ᡢ���)��
(2)��������ɿ���һ��������Ԫ����ɵ���________(�������ᡢ���)��
(3)��һ���û�ѧ����ʽ��ʾ��Ӧ��________����һ���û�ѧ����ʽ��ʾ��Ӧ��________��
(4)NH4NO3��Һ��NaOH��Һ�ܹ���Ӧ��ʵ������Ϊ������ˮ�н��������NH4+��OH�����������NH3��H2O����ϸ��ֽⷴӦ��������������ش�CaCl2��Һ��Na2CO3��Һ�ܹ���Ӧ��ʵ����������ˮ�н������________���������________��
(5)������(4)�л�õ���ʾ���ж����и���������ˮ��Һ���ܴ����������________��
A��Ba2+��SO42��
B��CO32����H+
C��H+��OH��
D��Na+��NO3��
������
|
����(1)������� ����(2)�� ����(3)Fe��CuSO4 ����(4)Ca2+��CO32����CaCO3 ����(5)D |

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������-����ʾ����֮���ܹ�������ѧ��Ӧ��
����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������-����ʾ����֮���ܹ�������ѧ��Ӧ�� ��2013?��ƽ��ģ�⣩����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ������ ��ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������-����ʾ����֮���ܹ�������ѧ��Ӧ���밴Ҫ��ش��������⣮
��2013?��ƽ��ģ�⣩����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ������ ��ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������-����ʾ����֮���ܹ�������ѧ��Ӧ���밴Ҫ��ش��������⣮ ����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������-����ʾ����֮���ܹ�������ѧ��Ӧ��
����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D�ֱ��ʾ��ͬ���Ļ������-����ʾ����֮���ܹ�������ѧ��Ӧ��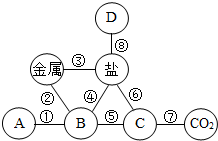 ����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ��������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D��ʾ������ᡢ��ε�ijһ�֣���-����ʾ����֮���ܹ�������ѧ��Ӧ��
����֪ʶ������ѧϰ���о���ѧ���õĿ�ѧ��������ͼ��С��ͬѧ�ڸ�ϰ���������ߵĻ�ѧ���ʡ�ʱ������֪ʶ���磬����A��B��C��D��ʾ������ᡢ��ε�ijһ�֣���-����ʾ����֮���ܹ�������ѧ��Ӧ��