题目内容
【题目】如下图所示,A-J是初中化学常见的物质。图中“→”表示转化关系,“一”表示相互能反应(部分反应物、生成物及反应条件已略去)。A、B、H常温下为气体,C、E为组成元素相同的氧化物。D、F、G为不同类别的化合物,其中F可以用于改良酸性土壤,G是常见的建筑材料,Ⅰ广泛应用于造纸、印染、纺织等工业。请回答下列问题:
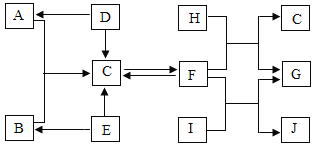
(1)写出符合物质G的一个化学式______。
(2)化学反应D→A基本反应类型为____。
(3)写出E→C反应的化学方程式_____。
(4)写出F和I的反应在生产生活中的用途____。
【答案】CaCO3 置换反应 2H2O2![]() 2H2O+O2 实验室制取少量烧碱
2H2O+O2 实验室制取少量烧碱
【解析】
F可以用于改良酸性土壤,知F是氢氧化钙;G是常见的建筑材料,可知G是碳酸钙;I广泛应用于造纸、印染、纺织等工业知I是碳酸钠,C、E为组成元素相同的氧化物,根据图表的信息可知E是过氧化氢;C是水;B是氧气;A和氧气在一定条件生成水,A是氢气;D、F、G为不同类别的化合物,D在一定条件下能生成氢气和水,可知D为硫酸(或盐酸);水在一定条件下能生成F,F在一定条件下也能生成水,F和碳酸钠能发生反应,知F是氢氧化钙;H常温下为气体且能和氢氧化钙反应生成水,H为二氧化碳,通过以上信息可知G为碳酸钙、J为氢氧化钠。
(1)G为碳酸钙,化学式为CaCO3;
(2)硫酸和锌反应生成硫酸锌和氢气,反应的化学方程式为:Zn+H2SO4=ZnSO4+H2↑,该反应的特征是单质与化合物反应生成单质和化合物,符合置换反应的特征;
(3)过氧化氢在二氧化锰的催化作用下生成水和氧气,反应的化学方程式为:2H2O2![]() 2H2O+O2↑;
2H2O+O2↑;
(4)氢氧化钙和碳酸钠反应生成氢氧化钠和碳酸钙沉淀,该反应用于实验室制取少量的烧碱。
故答案为:
(1)CaCO3;
(2)置换反应;
(3)2H2O2![]() 2H2O+O2↑;
2H2O+O2↑;
(4)实验室制取少量的烧碱。

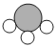
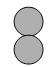
【题目】甲、乙、丙、丁表示4种物质,甲和乙在一定条件下反应生成丙和丁。其化学式和分子微观示意图如下表所示。下列叙述正确的是
物质 | 甲 | 乙 | 丙 | 丁 |
|
化学式 | NH3 | Cl2 | N2 | ? | |
分子微观示意图 |
|
|
|
|
A.反应前后分子个数不变
B.丁的化学式为HClN
C.该反应属于置换反应
D.若17g甲参加反应,则生成丁的质量为14g
【题目】某化学兴趣小组在老师的帮助下,开展了氢气的燃烧实验的相关探究:
(1)氢气燃烧的化学反应方程式为____________,在氢气点燃之前进行的操作是____________。
(2)作研究氢气的燃烧实验,需用试管收集不同体积比的氢气与空气的混合物,现用正确装置制取氢气,如何用试管(假设试管容积为10mL)收集氢气与空气体积比为4:1的混合气体,写出其操作方法:____________。
(3)用不同体积比的混合气体做氢气的燃烧实验,结果如表:
序号 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
氢气与空气体积比 | 9:1 | 8:2 | 7:3 | 5:5 | 3:7 | 1:9 | 0.5:9.5 |
点燃现象 | 安静燃烧 | 安静燃烧 | 弱的爆鸣声 | 强的爆鸣声 | 强的爆鸣声 | 弱的爆鸣声 | 不燃烧不爆鸣 |
分析上表信息,你对燃烧条件的新认识是可燃物能否燃烧除具备燃烧条件外,还与____________有关。