��Ŀ����
1�����Ҫ���б�ҵ�ˣ��뿪ĸУ֮�ʣ���Ҫ�������һ����ӡ����������ѧУ��һ��һľ�������Ӷ������������������Լ��Ķ���ʱ��������������Ʒ����A������ҵ�� ��B����ƻ�� ��C�������� ��D�������˵ı�
��E����������� ��F������������ƿ ��G���Ͼ�Ϳ��Һƿ
��1��Ӧ���롰�ɻ��������䡱����ACEFG������ţ���
��2���������ȥ�����������壨��Ҫ�ɷ���FeO3���Ļ�ѧ����ʽΪ6HCl+Fe2O3�T2FeCl3+3H2O��
��3������дһ��Ȱ�������꼶ͬѧ����У�����Ļ��������������������
���� ��1�����ݷ�����Ŀ����ü�ֵ���з�����
��2������ijɷ����������������ᷴӦ�����κ�ˮ�����ݷ���ʽ��дҪ����н��
��3���ɴ�������������Ϊ������������ڻ��������ķ�����н��
��� �⣺��1������ҵ��Ϊֽ�࣬���Ի������ã�����������ƿ����Ȫˮƿ���Ͼ�Ϳ��ҺƿΪ���ϣ����Ի������ã���������������������ڽ��������Ի������ã�ù��ĵ������ƻ�������ü�ֵ��
��2������ijɷ�Ϊ���������������ᷴӦ�����κ�ˮ����ѧ����ʽΪ��6HCl+Fe2O3�T2FeCl3+3H2O��
��3��ֻҪ�Ƿ��ϱ���У�����ļ��ɣ��磺�����������������𣻰������ǹ�ͬ�ļң�С��Ҳ�������������̤��������ǰ����������������С·�ȣ�
�𰸣�
��1��ACEFG��
��2��6HCl+Fe2O3�T2FeCl3+3H2O��
��3��������������������
���� ��2����Ҫ�ɷ���FeO3 ΪFe2O3

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ
16��ij���ӵĽṹʾ��ͼΪ ���������ǣ�������
���������ǣ�������
 ���������ǣ�������
���������ǣ�������| A�� | ԭ�� | B�� | ԭ���� | C�� | ������ | D�� | ������ |
20�����и������ʵ������뻯ѧʽһ�µ��ǣ�������
| A�� | ������KOH | B�� | ��ʯ��Ca��OH��2 | C�� | ��ʯ��CaO | D�� | �ɱ�CO |
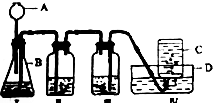 ������ʵ������ȡ������̼����֤�����ʵ�ʵ��װ��ͼ������װ����ʯ��ˮ������װ����ɫʯ����Һ�����ش��������⣮
������ʵ������ȡ������̼����֤�����ʵ�ʵ��װ��ͼ������װ����ʯ��ˮ������װ����ɫʯ����Һ�����ش��������⣮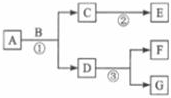 A��G�dz��л�ѧ������7�����ʣ�����������һ����������ɫ���壬��A��G������ͬ�Ľ���Ԫ�أ�������һ��������ת����ϵ��ͼ��ʾ�����в��ַ�Ӧ�������������ȥ�� �����������������ش����⣮
A��G�dz��л�ѧ������7�����ʣ�����������һ����������ɫ���壬��A��G������ͬ�Ľ���Ԫ�أ�������һ��������ת����ϵ��ͼ��ʾ�����в��ַ�Ӧ�������������ȥ�� �����������������ش����⣮