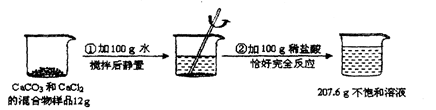��Ŀ����
ijͬѧΪ�˲ⶨ��ͭм����п��ͭ�γɵĺϽ���Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 50��0 | 50��0 | 50��0 | 50��0 |
| ȡϡ����������g�� | 40��0 | 80��0 | 120��0 | 160��0 |
| ��������������g�� | 0��4 | 0��8 | 1��0 | 1��0 |
�Լ��㣺
�Ÿ��ݲ�õ����ݷ�������1����Ʒ�� �������ʣ���ȫ��Ӧ�ˡ�
����ʽ�����ͭм��Ʒ�е�п������������
������ͼ�л�����50��0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
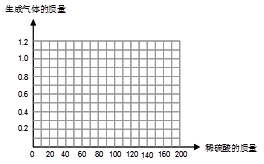
��1��ϡ���� ��2�֣�
��2��65% ��1�֣�
���������������1���ӱ��е����ݿ��Կ�����50g�Ͻ������Ժ�100gϡ���ᷴӦǡ����ȫ��Ӧ������1��0g���������Ե�һ������ϡ���ᷴӦ���ˣ�������ʣ�ࣻ�������Ĵν�����Ӧ���ˣ�ϡ������ʣ�ࣨ2���ȸ���1��0g�������������п������Ϊ32��5g������32��5g����50g�͵ó���Ʒ��п������������3����ϡ����Ϊ40gʱ������Ϊ0��4g��ϡ����Ϊ80gʱ������Ϊ0��8g���Դ����ƣ�ϡ����Ϊ100gʱ������Ϊ1��0g��ϡ�������100gʱ������Ȼ��1��0g������⼸����������������Ϳ�����
���㣺�������ݽ��з������ٽ��л�ѧ����ʽ�ļ���

Ϊ�˲ⶨij��ĩ״��ͭ��ͭ��п�Ͻ���Ʒ��ͭ����������������ͬѧȡ10g��ͭ��Ʒ�����ձ��У���ȡ40gϡ������Ĵμ����ձ��У�����ַ�Ӧ��ʵ���������£�
| | ��һ�� | �ڶ��� | �ڶ��� | ���Ĵ� |
| ����ϡ����������g�� | 10g | 10g | 10g | 10g |
| ʣ������������g�� | 8.7g | 7.4g | 6.1g | 5.45g |
��1����С��ͬѧҪ����������Ϊ36��5%Ũ�������������40��ϡ�Σ�����Ũ���������Ϊ_ ��
��2��������Ӧ�Ļ�ѧ����ʽΪ ��
��3���г��������������������X���ı���ʽ ��
��4�����һ�η�Ӧ��������Һ�м���8.74gˮ����������Һ����������Ϊ ��
��5��ij����Ҫ���Ƶ�2000t��ͭ����Ҫ������50%�Ļ�ͭ����Ҫ�ɷ���Cu2S��������Ϊ ��
���Ȼ��ƺ�̼����泥�NH4HCO3�����Ʊ�̼�����ƺ��Ȼ�泥�NH4Cl�����÷�Ӧ�ɱ�ʾΪ��NaCl+NH4HCO3�TNaHCO3+NH4Cl��
20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100gˮ�м���11.7g NaCl
��15.8g NH4HCO3������㣺
��1�������ϴ���Һ���������������Ϊ����g��
��2����Ӧ��������Һ������NH4Cl�����������Ƕ��٣���д������ʽ���ɣ���
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣮
| ���� | NaCl | NH4HCO3 | NH4Cl | NaHCO3 |
| �ܽ��/g | 36.0 | 21.6 | 37.2 | 9.6 |
���ݺ�°��Ƽԭ����ʵ�����Ʊ�����(Na2CO3)����Ҫ�����ǣ��������ƺõı���NaCl��Һ�����ձ��м��ȣ������¶���30-35�棬�����·���������ϸ��NH4HCO3���壬������Ϻ�������30���ӣ����á����˵�NaHCO3���塣����������ˮϴ�ӳ�ȥ���ʣ�����������ת���������У�����2Сʱ����Na2CO3���塣
�������ڲ�ͬ�¶��µ��ܽ�ȣ�g����
 �¶� �¶� �ܽ�� �� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | �� | ���� | ���� | ���� |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | ���� |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
|
��1������NaCl��Һ�м���NH4HCO3���壬�����˸��ֽⷴӦ������NaHCO3���壬д�����з�����Ӧ�Ļ�ѧ����ʽ ��
��2��������Ϻ�������30���ӣ�Ŀ���� ��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ���ʣ�������һ�����е������� ���Ի�ѧʽ��ʾ����
��3�����˵�NaHCO3��������Һ�л���һ������NaHCO3�ܽ���ˮ�У���ʱ������Һ�м���һ������ ��������һ��������ʹNaCl��Һѭ��ʹ�á�
��4��ijС�մ���Ʒ�л�������̼���ƣ�ȡҩƷ2.0g���ȵ��������ټ���Ϊֹ�����ռ���������̼����0.22L��������̼���ܶ�Ϊ2.0g/L��������Ⱥ��ʣ������м���һ������������Ϊ3.65%��ϡ���ᣬʹ��ǡ����ȫ��Ӧ�������ĸ�ϡ����������Ƕ��ٿˣ�