��Ŀ����
��5�֣���������ϡ��Һ�����ΪA��0.0400%������������Һ�����ΪB��0.365%�����ᡣ���豾�����漰���ĸ���ϡ��Һ���ܶȾ�����Ϊ1.00g��mL-1����ÿ����Һ���������Ϊ0. 05mL���Խ�����и�С�⡣
��1��ǡ����ȫ�к�20. 0gA��Һ�������B��Һ���ٿˣ�
��2����ʢ��20. 0mLA��Һ����ƿ�еμ�2�η�̪��Һ������ƿ�л�������10. 0mLB��Һ���ߵ�������ֻ�Ϻ���Һ����ɫ����ȡ����ɫ���Һ3.00mL��һ֧�Թ��ڣ������Թ��ڵμ�1��A��Һ����ͨ������˵����ʱ�Թ�����Һ���ֵ���ɫ��
��1��ǡ����ȫ�к�20. 0gA��Һ�������B��Һ���ٿˣ�
��2����ʢ��20. 0mLA��Һ����ƿ�еμ�2�η�̪��Һ������ƿ�л�������10. 0mLB��Һ���ߵ�������ֻ�Ϻ���Һ����ɫ����ȡ����ɫ���Һ3.00mL��һ֧�Թ��ڣ������Թ��ڵμ�1��A��Һ����ͨ������˵����ʱ�Թ�����Һ���ֵ���ɫ��
��1��2.00g�� ��2����Һ����ɫ
�����������֪�����������ƣ�20.0g��0.0400%=0.008g�� 0.365%�����10. 0mLB��Һ���Ȼ����������10. 0mLB��Һ�����Һ3.00mL��δ֪������l)ǡ����ȫ�к�20. 0gA��Һ�������B��Һ����������2���Թ�����Һ����ɫ��
�⣺��1��ǡ����ȫ�к�20. 0gA��Һ�������B��Һ������Ϊx
HCl + NaOH==NaCl+H2O
36.5 40
0.365%x 0.008g
 =
=
x=2.00g
��2��20. 0mL����������Һ�к���������������Ϊ8g����Ϊǡ����ȫ�к�20. 0gA��Һ����0.365%�����������Ϊ2.00g��3mL�������Ȼ��������Ϊ��3mL��30mL-1��8g��0.365%=2.92g��10-3g��
��1��A��Һ�е������������к�1�Ȼ��������Ϊy��
HCl + NaOH==NaCl+H2O
36.5 40
y 0.05g��0.04%
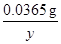 =
=
y-1.825g��10-3g<2.92g��10-3g;
����������ʣ�࣬��Һ����ɫ��

��ϰ��ϵ�д�
�����Ŀ


