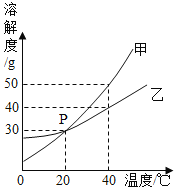
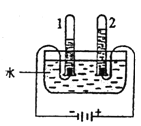
��Ŀ����
����Ŀ��A��H���dz��л�ѧ�������ʡ�����A��B�����Ԫ����ͬ���ҳ����¶�����ɫҺ�壻H����ɫ������X��Y�������������X�Ǻ�ɫ��ĩ״���壬Y������ʳƷ�������Z�Ǻ�ɫ���塣����֮������ͼת����ϵ��

����������Ϣ���ش��������⣺
��1����Ӧ����X��________���á�
��2����Ӧ�ڵĻ�ѧ����ʽ___________________________________��
��3����Ӧ�ݵĻ�ѧ����ʽ___________________________________��
��4����ת����ϵ��û���漰���Ļ�����Ӧ������________��Ӧ��
���𰸡���1���� ����2��CaO+ H2O�� Ca(OH)2 ����3��CuSO4 + Ca(OH)2 ��CaSO4+ Cu(OH)2������4���û�
��������
�������������A��B�����Ԫ����ͬ���ҳ����¶�����ɫҺ�壻˵��A�ǹ������⣬������ˮ����X�Ƕ���������C��������Z�Ǻ�ɫ���壬��H����ɫ������˵��Z��ͭ����E������ͭ����������ͭ������һ���������ˮ��Ӧ������������ƣ��������������ƣ������������⡣

��ϰ��ϵ�д�
 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
�����Ŀ