��Ŀ����
��2013?������һģ����Դ���������������ᷢչ������أ�
��1�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ�
��2�����й�����Դ�������仯�������У���ȷ����
A�����ͼ���ȼ�ϣ��������õ��ܼ�
B���ڻ�ѧ��Ӧ��ֻ��ȼ�ղ��ܷų�����
C���������õ���������ͨ����ѧ��Ӧ��õ�
D��ú�Ƴɡ�����ú����Ϊ������������ĽӴ������ʹ��ȼ�ճ��
��3��Ϊ��ֹ������̼�����ŷŵ������У���ʵ�������о�������NaOH��Һ��������CO2������ͼ��ͼ��ʾ����������������δ�������
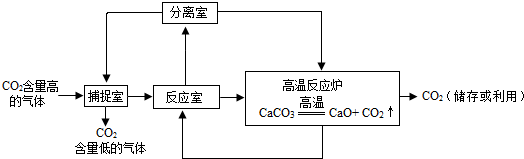
�ٲ����ڷ�����Ӧ�Ļ�ѧ����ʽΪ
�ڷ�Ӧ���ڷ�����Ӧ�Ļ�ѧ����ʽΪ
��1�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ�
��Ȼ��
��Ȼ��
����2�����й�����Դ�������仯�������У���ȷ����
AD
AD
��A�����ͼ���ȼ�ϣ��������õ��ܼ�
B���ڻ�ѧ��Ӧ��ֻ��ȼ�ղ��ܷų�����
C���������õ���������ͨ����ѧ��Ӧ��õ�
D��ú�Ƴɡ�����ú����Ϊ������������ĽӴ������ʹ��ȼ�ճ��
��3��Ϊ��ֹ������̼�����ŷŵ������У���ʵ�������о�������NaOH��Һ��������CO2������ͼ��ͼ��ʾ����������������δ�������

�ٲ����ڷ�����Ӧ�Ļ�ѧ����ʽΪ
CO2+2NaOH�TNa2CO3+H2O
CO2+2NaOH�TNa2CO3+H2O
���ڷ�Ӧ���ڷ�����Ӧ�Ļ�ѧ����ʽΪ
CaO+H2O�TCa��OH��2��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
CaO+H2O�TCa��OH��2��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
����������1�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ����
��2��A�����ͼ���ȼ�ϣ��������õ��ܼ���
B��ȼ���ܷų�һ�������������ڲ�ȼ��ʱ�п���Ҳ��ų�������
C����ѧ��Ӧ�������ȡ�����ķ�ʽ֮һ��
D��������ú����������������ĽӴ���������ȼ�գ�
��3���ٸ��ݷ�Ӧ��������Լ������غ㶨��д������ʽ��
�ڸ���ͼʾ��Ϣ�ͷ�Ӧ��ϵ�������
��2��A�����ͼ���ȼ�ϣ��������õ��ܼ���
B��ȼ���ܷų�һ�������������ڲ�ȼ��ʱ�п���Ҳ��ų�������
C����ѧ��Ӧ�������ȡ�����ķ�ʽ֮һ��
D��������ú����������������ĽӴ���������ȼ�գ�
��3���ٸ��ݷ�Ӧ��������Լ������غ㶨��д������ʽ��
�ڸ���ͼʾ��Ϣ�ͷ�Ӧ��ϵ�������
����⣺��1�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ����
��2��A��������ȼ�ϣ������ܼ�������ȷ��
B��ȼ���ܷų�һ�������������ڲ�ȼ��ʱ�п���Ҳ��ų���������������������ˮͬ����ų��������ʴ�ѡ�����
C����ѧ��Ӧ�������ȡ�����ķ�ʽ֮һ�����ܺ��ܵ�Ҳ�������ȡ�����ķ�ʽ���ʴ�ѡ�����
D��������ú����������������ĽӴ���������ȼ�գ��ʴ�ѡ����ȷ��
��3���ٶ�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ�����Բ����ڷ�Ӧ�Ļ�ѧ����ʽΪ��C02+2NaOH�TNa2C03+H20��
���������֪���ڲ��������ն�����̼����Һ������������Һ���������м��������ƺ�Y��Һ������������������Һ�������ƿ���ˮ��Ӧ�����������ƣ�������������̼������Һ��Ӧ����������������Һ��̼��Ƴ��������ڷ�Ӧ�������ڷ����ķ�Ӧ����ʯ����ˮ������������̼���Ƶķ�Ӧ��
�ʴ�Ϊ����1����Ȼ����
��2��AD��
��3����CO2+2NaOH�TNa2CO3+H2O��
��CaO+H2O�TCa��OH��2�� Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��2��A��������ȼ�ϣ������ܼ�������ȷ��
B��ȼ���ܷų�һ�������������ڲ�ȼ��ʱ�п���Ҳ��ų���������������������ˮͬ����ų��������ʴ�ѡ�����
C����ѧ��Ӧ�������ȡ�����ķ�ʽ֮һ�����ܺ��ܵ�Ҳ�������ȡ�����ķ�ʽ���ʴ�ѡ�����
D��������ú����������������ĽӴ���������ȼ�գ��ʴ�ѡ����ȷ��
��3���ٶ�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ�����Բ����ڷ�Ӧ�Ļ�ѧ����ʽΪ��C02+2NaOH�TNa2C03+H20��
���������֪���ڲ��������ն�����̼����Һ������������Һ���������м��������ƺ�Y��Һ������������������Һ�������ƿ���ˮ��Ӧ�����������ƣ�������������̼������Һ��Ӧ����������������Һ��̼��Ƴ��������ڷ�Ӧ�������ڷ����ķ�Ӧ����ʯ����ˮ������������̼���Ƶķ�Ӧ��
�ʴ�Ϊ����1����Ȼ����
��2��AD��
��3����CO2+2NaOH�TNa2CO3+H2O��
��CaO+H2O�TCa��OH��2�� Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
���������⿼����ѧ������ͼʾ����д����ʽ������������ʱ����Ҫ��������ͼ��ȷ���ʵ��Ʊ����̣�����ȷ�������Ļ�ѧ��Ӧ���Ӷ�д����Ӧ�ķ���ʽ���������ܺܺõĿ���ѧ����������������������

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ
 ��2013?������һģ��ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮
��2013?������һģ��ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�飮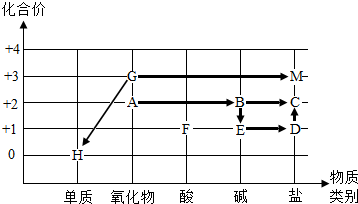 ��2013?������һģ��ͬѧ��������������������ij��Ԫ�صĻ��ϼ۹����˳��л�ѧ�������ʼ��ת����ϵ����ͼ��ͼ�С�������ʾ���ʼ��ת������
��2013?������һģ��ͬѧ��������������������ij��Ԫ�صĻ��ϼ۹����˳��л�ѧ�������ʼ��ת����ϵ����ͼ��ͼ�С�������ʾ���ʼ��ת������ ��2013?������һģ��ʵ�����ù�����ϡ����ʹ���ʯ��ȡCO2��ȡ50g��Ӧ�����Һ����ε���̼������Һ����õ���̼������Һ�����������������������ϵ��ͼ��ʾ��
��2013?������һģ��ʵ�����ù�����ϡ����ʹ���ʯ��ȡCO2��ȡ50g��Ӧ�����Һ����ε���̼������Һ����õ���̼������Һ�����������������������ϵ��ͼ��ʾ��