��Ŀ����
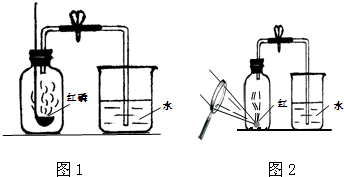
 ��ͼ1��ʾ��ʵ������ȡ���ռ���һƿC02����ƿ�е������ʯ��ˮ��Ѹ����Ԥ�Ȱ��һ��С�������Ƥ��������
��ͼ1��ʾ��ʵ������ȡ���ռ���һƿC02����ƿ�е������ʯ��ˮ��Ѹ����Ԥ�Ȱ��һ��С�������Ƥ����������1����װ�ã����Թ۲쵽С���� ������͡�������С�������ޱ仯��֮һ�����������ָ������ԭ��
��2��ʵ����˳�ƿ�а�ɫ��������ձ������ձ�����εμ�������������Ϊ1.46%��ϡ���ᣬ�ձ��а�ɫ�����������������ϡ�����������ϵ������ͼ��ʾ�����������ش��������⣺
�ٵ�����1.46%��ϡ����70gʱ����ͼ2��B�㣩���ձ�����Һ�����������ǣ� ���ѧʽ��
�ڵ�����1.46%��ϡ����50gʱ����ͼ2��A�㣩���ձ������ò�������Һ����������������������O��Olg��
���𰸡��������������е�֪ʶ���з�����������̼�����������Ʒ�Ӧ����̼��Ƴ�����ˮ�����屻���ģ����ܱ������ڵ�ѹǿ��С��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ַ�Ӧ���������ɼ���������Һ��������
����⣺��1��������̼�����������Ʒ�Ӧ����̼��ƺ�ˮ��������̼�����ģ���ƿ�ڵ�ѹǿС�ڴ���ѹ���ڴ���ѹ�������£�С��������ͣ�������ͣ�������̼���������Ʒ�Ӧ�������壬����ƿ�ڵ�ѹǿС�ڴ���ѹ��
��2��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��������70g����ʱ��̼����Ѿ���ȫ��Ӧ���������������Һ�к��е��������Ȼ��ƺ��Ȼ��⣬���HCl��CaCl2��
��3��������1.46%��ϡ����50gʱ��̼��ƺ�����ǡ����ȫ��Ӧ����̼��Ƶ�������x�����ɵĶ�����̼��������y������
CaCO3+2 HCl=CaCl2+H2O+CO2��
100 73 44
x 1.46%×50g y

x=1g y=0.44g
������Һ������Ϊ��1g+50g-0.44g=50.56g
�����ò�������Һ������Ϊ50.56g��
���������⿼�������������������̼�ķ�Ӧ�Լ�������ѹǿ֪ʶ�Ľ�Ϻ��ݻ�ѧ����ʽ�ļ��㣬��ɴ��⣬�����������е�֪ʶ���ͼʾ���У�
����⣺��1��������̼�����������Ʒ�Ӧ����̼��ƺ�ˮ��������̼�����ģ���ƿ�ڵ�ѹǿС�ڴ���ѹ���ڴ���ѹ�������£�С��������ͣ�������ͣ�������̼���������Ʒ�Ӧ�������壬����ƿ�ڵ�ѹǿС�ڴ���ѹ��
��2��̼����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��������70g����ʱ��̼����Ѿ���ȫ��Ӧ���������������Һ�к��е��������Ȼ��ƺ��Ȼ��⣬���HCl��CaCl2��
��3��������1.46%��ϡ����50gʱ��̼��ƺ�����ǡ����ȫ��Ӧ����̼��Ƶ�������x�����ɵĶ�����̼��������y������
CaCO3+2 HCl=CaCl2+H2O+CO2��
100 73 44
x 1.46%×50g y

x=1g y=0.44g
������Һ������Ϊ��1g+50g-0.44g=50.56g
�����ò�������Һ������Ϊ50.56g��
���������⿼�������������������̼�ķ�Ӧ�Լ�������ѹǿ֪ʶ�Ľ�Ϻ��ݻ�ѧ����ʽ�ļ��㣬��ɴ��⣬�����������е�֪ʶ���ͼʾ���У�

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ



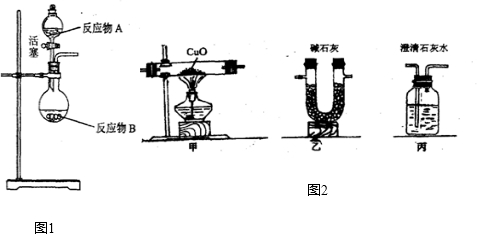
 NH3?H2O
NH3?H2O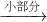 NH4++OH-����ˮ����Ҫ��������NH3?H2O��NH3?H2O���ȶ��������������ֽ�ų����������⣬������Ӧ���淴Ӧ��������ͬ��������ͬʱ����������������еķ�Ӧ�������ӷ�Ӧ���Ũ�ȣ��ܴ�ʹ��Ӧ���ҽ��У����Ӳ����Ũ�ȣ��ܴ�ʹ��Ӧ������У�ijͬѧ��Ũ��ˮ����һ�ֹ���������ȡ��������Ӧ��AΪŨ��ˮ����Ӧ��BΪ______���壬�ø÷�����ȡ������ԭ��Ϊ______�������ֱ�������
NH4++OH-����ˮ����Ҫ��������NH3?H2O��NH3?H2O���ȶ��������������ֽ�ų����������⣬������Ӧ���淴Ӧ��������ͬ��������ͬʱ����������������еķ�Ӧ�������ӷ�Ӧ���Ũ�ȣ��ܴ�ʹ��Ӧ���ҽ��У����Ӳ����Ũ�ȣ��ܴ�ʹ��Ӧ������У�ijͬѧ��Ũ��ˮ����һ�ֹ���������ȡ��������Ӧ��AΪŨ��ˮ����Ӧ��BΪ______���壬�ø÷�����ȡ������ԭ��Ϊ______�������ֱ�������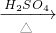 CO2��+CO��+H2O������ͼ2��ʾ�ķ���װ�ý���ʵ�飬����Ҫ���ӵ������оƾ��ơ���Ȧ��______��ijͬѧΪ�����������е�CO�������û����������ͨ���ҡ������ס�����β����ȼ�����е�һ��ͨ������������______��
CO2��+CO��+H2O������ͼ2��ʾ�ķ���װ�ý���ʵ�飬����Ҫ���ӵ������оƾ��ơ���Ȧ��______��ijͬѧΪ�����������е�CO�������û����������ͨ���ҡ������ס�����β����ȼ�����е�һ��ͨ������������______��
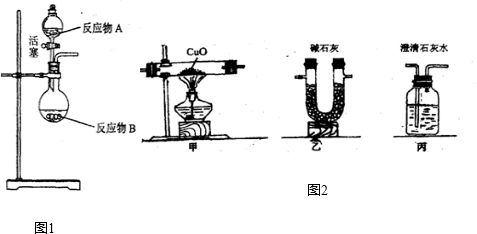
 NH3?H2O
NH3?H2O NH4++OH-����ˮ����Ҫ��������NH3?H2O��NH3?H2O���ȶ��������������ֽ�ų����������⣬������Ӧ���淴Ӧ��������ͬ��������ͬʱ����������������еķ�Ӧ�������ӷ�Ӧ���Ũ�ȣ��ܴ�ʹ��Ӧ���ҽ��У����Ӳ����Ũ�ȣ��ܴ�ʹ��Ӧ������У�ijͬѧ��Ũ��ˮ����һ�ֹ���������ȡ��������Ӧ��AΪŨ��ˮ����Ӧ��BΪ ���壬�ø÷�����ȡ������ԭ��Ϊ �������ֱ�������
NH4++OH-����ˮ����Ҫ��������NH3?H2O��NH3?H2O���ȶ��������������ֽ�ų����������⣬������Ӧ���淴Ӧ��������ͬ��������ͬʱ����������������еķ�Ӧ�������ӷ�Ӧ���Ũ�ȣ��ܴ�ʹ��Ӧ���ҽ��У����Ӳ����Ũ�ȣ��ܴ�ʹ��Ӧ������У�ijͬѧ��Ũ��ˮ����һ�ֹ���������ȡ��������Ӧ��AΪŨ��ˮ����Ӧ��BΪ ���壬�ø÷�����ȡ������ԭ��Ϊ �������ֱ�������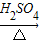 CO2��+CO��+H2O������ͼ2��ʾ�ķ���װ�ý���ʵ�飬����Ҫ���ӵ������оƾ��ơ���Ȧ�� ��ijͬѧΪ�����������е�CO�������û����������ͨ���ҡ������ס�����β����ȼ�����е�һ��ͨ������������ ��
CO2��+CO��+H2O������ͼ2��ʾ�ķ���װ�ý���ʵ�飬����Ҫ���ӵ������оƾ��ơ���Ȧ�� ��ijͬѧΪ�����������е�CO�������û����������ͨ���ҡ������ס�����β����ȼ�����е�һ��ͨ������������ ��