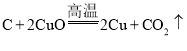��Ŀ����
����Ŀ���й��Ŵ��Ĵ���֮һ�����ڻ�ҩ������ľ̿(C)�����(S)�������(KNO3)��һ��������϶��ɡ�
(1)���ࡣ���й������ڻ�ҩ��˵����ȷ����__________��
a ���ڻ�ҩ�������ڻ�ҩ����������
b ���ڻ�ҩ���е�KNO3���ڸ��Ϸ���
c ���ڻ�ҩ���е�C��S���ٱ��ָ��ԵĻ�ѧ����
(2)�仯�����ڻ�ҩ����ըʱ��������Ҫ��Ӧ�ǣ�![]() ��
��
�����������غ㶨�ɣ��ո���ȱ�ٵ�������____________(�ѧʽ)��
�����ڻ�ҩ����ըʱ���ŵ��̱ǵĻ�ҩζ������Ϊ��ըʱ�������������������������ɡ����ڿ�����ȼ�յĻ�ѧ����ʽΪ___________��
(3)�Ʊ����Ŵ���������(����Ca(NO3)2������NaCl��)�Ͳ�ľ��(����K2CO3)��ԭ����ȡKNO3��ij��ѧ��ȤС�����������ʵ�����̣�
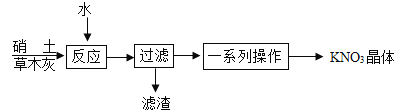
������Ӧ�������еĻ�ѧ����ʽΪ____________��
�������������������õ��IJ����������ձ�����������___________��
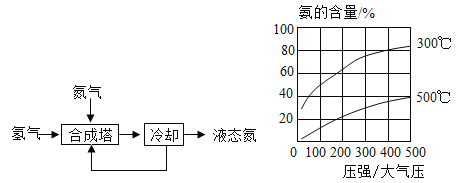
����ͼ������غ��Ȼ��Ƶ��ܽ�����ߡ���һϵ�в�������������������Ũ��������ȴ��һ���¶ȡ��������Ȳ��衣��������ȴ��һ���¶����ܻ�ô��Ƚϸߵ�����ؾ����ԭ����____________��

(4)��չ���ִ������������ʹ�õ�����ըҩ����Ҫ�ɷ�Ϊ��������(C3H5O9N3)��
�������������������ܻ�����������һ�ֵ�����������������Ľ�ʹ�����������е�Ϊ+2�ۣ��仯ѧʽΪ____________���ϳ��������͵Ļ�ѧ����ʽΪ![]() ������46kg����(C3H8O3)���������ᷴӦ�������������ɶ����������ͣ�__________(д���������)
������46kg����(C3H8O3)���������ᷴӦ�������������ɶ����������ͣ�__________(д���������)
���𰸡�b CO2 S+O2![]() SO2 Ca(NO3)2+K2CO3=CaCO3��+2KNO3 ©�� KNO3���ܽ�������¶ȵĽ��Ͷ�������С���Ȼ����������ܽ�����¶�Ӱ�첻�������������� NO �⣺��46kg���ͺ��������ᷴӦ�������������������͵�����Ϊx
SO2 Ca(NO3)2+K2CO3=CaCO3��+2KNO3 ©�� KNO3���ܽ�������¶ȵĽ��Ͷ�������С���Ȼ����������ܽ�����¶�Ӱ�첻�������������� NO �⣺��46kg���ͺ��������ᷴӦ�������������������͵�����Ϊx
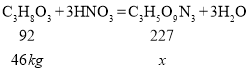
![]()
x=113.5kg
��46kg���ͺ����������ᷴӦ�����������������͵�����Ϊ113.5kg
��������
(1)
a�����ڻ�ҩ������ľ̿(C)�����(S)�������(KNO3)��һ��������϶��ɡ����ڻ����ʴ���
b�����ڻ�ҩ���е�KNO3ͬʱ���м�Ԫ�غ͵�Ԫ�أ����ڸ��Ϸ��ϣ�����ȷ��
c�����ڻ�ҩ���е�C��S���ָ��ԵĻ�ѧ���ʣ�ԭ���ǻ�ѧ�仯�е���С���ӣ��ʴ���
��ѡb
(2)��
�����������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵ����ࡢ���������䣬��ϻ�ѧ����ʽ��![]() ����Ӧ���к�1����ԭ�ӡ�2����ԭ�ӡ�2����ԭ�ӡ�3��̼ԭ�ӡ�6����ԭ�ӣ��������к�2����ԭ�ӡ�1����ԭ�ӡ�2����ԭ�ӣ����������л�Ӧ��3��̼ԭ�ӡ�6����ԭ�ӣ��ʿո���ȱ�ٵ�������CO2��
����Ӧ���к�1����ԭ�ӡ�2����ԭ�ӡ�2����ԭ�ӡ�3��̼ԭ�ӡ�6����ԭ�ӣ��������к�2����ԭ�ӡ�1����ԭ�ӡ�2����ԭ�ӣ����������л�Ӧ��3��̼ԭ�ӡ�6����ԭ�ӣ��ʿո���ȱ�ٵ�������CO2��
�����ڿ�����ȼ�����ɶ��������仯ѧ����ʽΪS+O2![]() SO2��
SO2��
(3)
������Ӧ������������ƺ�̼��ط�Ӧ����̼��Ƴ���������أ��仯ѧ����ʽΪCa(NO3)2+K2CO3=CaCO3��+2KNO3��
�������������������õ��IJ����������ձ�����������©����
�۸���ͼ����ʾ���ܽ�����ߣ�����ȴ��һ���¶����ܻ�ô��Ƚϸߵ�����ؾ����ԭ����KNO3���ܽ�������¶ȵĽ��Ͷ�������С���Ȼ����������ܽ�����¶�Ӱ�첻�������������塣
(4)
���������е�Ԫ��Ϊ+2�ۣ�Ϊʹ�仯������������ϼ۴�����Ϊ�㣬���仯ѧʽΪNO��
�ڽ⣺��46kg���ͺ��������ᷴӦ�������������������͵�����Ϊx
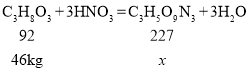
![]()
x=113.5kg
��46kg���ͺ����������ᷴӦ�����������������͵�����Ϊ113.5kg

 ��ս�п�����ϵ�д�
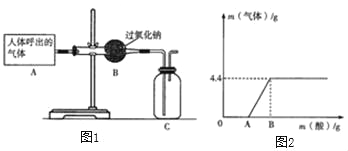
��ս�п�����ϵ�д�����Ŀ��ijͬѧ�ڰ���ʵ��Ա������ѧ�Լ�ʱ������һƿ��ǩ��ȱ����ɫ��Һ����ͼ����ʾ������ʵ��Ա������֪ԭƿ��Һ�е����ʿ�����NaCl��NaOH��Na2CO3��NaHCO3�е�һ�֣�Ϊȷ����Һ�е����ʣ�����Ʋ�����������̽�����
�����ϲ��ģ������������ʵ������Ϣ���£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ��/g | 36 | 109 | 21.5 | 9.6 |
������ϡ��Һ��pH | 7 | 13 | 11 | 9 |
��ش��������⣺
��ʵ��̽��1��
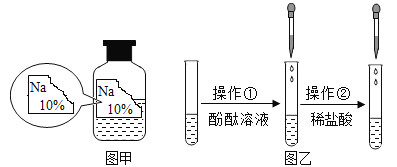
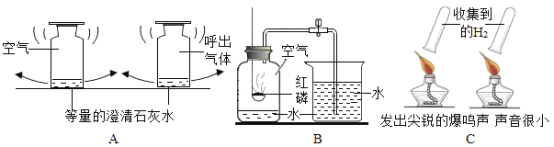
����ͼ����ʾ���ڲ����ٺ��ȷ�����ʲ���NaCl����ʵ������Ӧ��______���ڽ��в�����ʱ����ɫ��ζ����������ɴ��ֿ��ų���������__________��
��̽�����ۣ������������ʵ������Ϣ������Ϊ����Һ�е����ʿ������������������е�______������ж�������__________��������̽����������ȷ�ģ������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________��
���������ɣ���ͬѧ��Ϊ���Ϸ��������ܣ���Ҫ��һ��ʵ��ȷ���������ֽ���������̽����
��ʵ��̽��2��������ٺ��Թ��е���Һ�еμӹ���CaC12��Һ�����ԣ�����ַ�Ӧ���Թ�����Һ��ɫ���䣬���а�ɫ����������
���ó����ۣ�ͨ��ʵ��̽��2��ȷ��ԭƿ��Һ�е�����Ӧ����_________��
����˼��չ����ɸ���Һ��������ʵ�������ԭ����______________���û�ѧ����ʽ��ʾ����