��Ŀ����
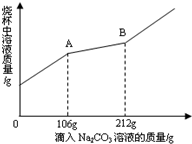
̼���ƣ�Na2CO3���׳��մ���һ�ְ�ɫ���壬�㷺���ڲ�������ֽ����֯��ϴ�Ӽ��������ȣ�������ϡ������ᷴӦ����CO2����Na2CO3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4�TNa2SO4+CO2��+H2O���ֽ�100gNa2CO3�����ĩ������Na2SO4���ʣ�����202gˮ�У���ȫ�ܽ⣬�ټ���100mLϡ���ᣨ�ܶ�Ϊ1.2g/mL����ǡ����ȫ��Ӧ��ʹ����ȫ���ݳ��������ĩ�����������CO2����Ĺ�ϵ��ͼ����״���£�CO2���ܶ�Ϊ2g/L����ͨ�����㣺
��1������CO2��������______g��
��2����100mLϡ���������ʵ������ͷ�Ӧ��������Һ����������������

��1������CO2��������______g��
��2����100mLϡ���������ʵ������ͷ�Ӧ��������Һ����������������

��1����ͼ���֪������̼�����Ϊ11L����m��CO2��=11L��2g/L=22g��
��2����100mL������Һ��H2SO4������Ϊx��100g��Ϲ����ĩ�к�Na2CO3������Ϊy������Na2SO4������Ϊz��
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106����98�� 142�� 44
y����x�� z�� 22g
=
=
=
��ã�x=49g��y=53g��z=71g
��Ϲ����ĩ�к�Na2SO4��������100g-53g=47g
��Ӧ��������Һ��������100g+202g+��100ml��1.2g/mL��-22g=400g
��Ӧ���������ʵ�������47g+71g=118g
��Ӧ��������Һ������������
��100%=29.5%
�ʴ�Ϊ����1��22��
��2����100mLϡ���������ʵ�������49g����Ӧ��������Һ��������������Ϊ29.5%��
��2����100mL������Һ��H2SO4������Ϊx��100g��Ϲ����ĩ�к�Na2CO3������Ϊy������Na2SO4������Ϊz��
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106����98�� 142�� 44
y����x�� z�� 22g
| 106 |
| y |
| 98 |
| x |
| 142 |
| z |
| 44 |
| 22g |
��ã�x=49g��y=53g��z=71g
��Ϲ����ĩ�к�Na2SO4��������100g-53g=47g
��Ӧ��������Һ��������100g+202g+��100ml��1.2g/mL��-22g=400g
��Ӧ���������ʵ�������47g+71g=118g
��Ӧ��������Һ������������
| 118g |
| 400g |
�ʴ�Ϊ����1��22��
��2����100mLϡ���������ʵ�������49g����Ӧ��������Һ��������������Ϊ29.5%��

��ϰ��ϵ�д�
 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ