��Ŀ����
ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ��� �����١��ڵ��ܿ�����ʱ��B�п�����ʵ�������ǣ� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��

�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
���ݱ���������������㣺
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��

�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
| ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1.54 | 3.08 | 4.4 | m |
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
ʯ����Һ ʯ����Һ����ɫ���ɫ CO2 + H2O ="==" H2 CO3
��ɫ����ʧ�ֱ���ɫ H2 CO3 ="==" H2 O + CO2��
��1����һ������ ��2��4.4
ע������ÿ�ո�1�֣���7��
��3���⣺�� ����1.54g CO2 ��Ҫ̼�������Ϊx��0.5�֣�
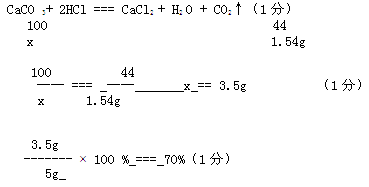
���ԣ�0.5�֣��������ⷨ��������Ҳ���֣�
��ɫ����ʧ�ֱ���ɫ H2 CO3 ="==" H2 O + CO2��
��1����һ������ ��2��4.4
ע������ÿ�ո�1�֣���7��
��3���⣺�� ����1.54g CO2 ��Ҫ̼�������Ϊx��0.5�֣�
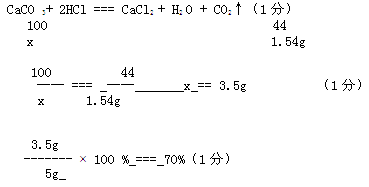
���ԣ�0.5�֣��������ⷨ��������Ҳ���֣�
��1��������̼����ˮ��Ӧ����̼�ᣬ����������ʹ��ɫʯ���죬���Է�Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O=H2CO3��
��2��������̼����ˮ��Ӧ����̼�ᣬ̼����ʹ��ɫ��ʯ����Һ��죮̼��ȶ��������ֽ⣬��˺�ɫ��Һ�ֻ�����ɫ ���Է�Ӧ�Ļ�ѧ����ʽΪH2 CO3 ="==" H2 O + CO2��
��1����3��ʵ��50gϡ����������ʯ��ʯû��ȫ��Ӧ����4��ʵ��ʯ��ʯ���ӵ�20gʱ��50gϡ������Ȼ�������ʯ��ʯû����ȫ��Ӧ��
�ʴ�Ϊ��1��2��
��2����ʯ��ʯ����Ϊ15gʱ������50gϡ�����������ʯ��ʯû��ȫ��Ӧ�����ԣ�ʯ��ʯ���ӵ�20gʱ��50gϡ������Ȼ�����㣬���Դ�ʱ����������̼��������ȻΪ4.4g��Ҳ����mֵΪ4.4g��
�ʴ�Ϊ��4.4g��
��3���⣺�� ����1.54g CO2 ��Ҫ̼�������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
5g��x 1.54g
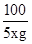 =
=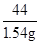
��֮�ã�x=70%
�𣺸�����ʯ��ʯ����̼��Ƶ���������Ϊ70%��
��2��������̼����ˮ��Ӧ����̼�ᣬ̼����ʹ��ɫ��ʯ����Һ��죮̼��ȶ��������ֽ⣬��˺�ɫ��Һ�ֻ�����ɫ ���Է�Ӧ�Ļ�ѧ����ʽΪH2 CO3 ="==" H2 O + CO2��
��1����3��ʵ��50gϡ����������ʯ��ʯû��ȫ��Ӧ����4��ʵ��ʯ��ʯ���ӵ�20gʱ��50gϡ������Ȼ�������ʯ��ʯû����ȫ��Ӧ��
�ʴ�Ϊ��1��2��
��2����ʯ��ʯ����Ϊ15gʱ������50gϡ�����������ʯ��ʯû��ȫ��Ӧ�����ԣ�ʯ��ʯ���ӵ�20gʱ��50gϡ������Ȼ�����㣬���Դ�ʱ����������̼��������ȻΪ4.4g��Ҳ����mֵΪ4.4g��
�ʴ�Ϊ��4.4g��
��3���⣺�� ����1.54g CO2 ��Ҫ̼�������Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
5g��x 1.54g
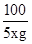 =
=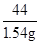
��֮�ã�x=70%
�𣺸�����ʯ��ʯ����̼��Ƶ���������Ϊ70%��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
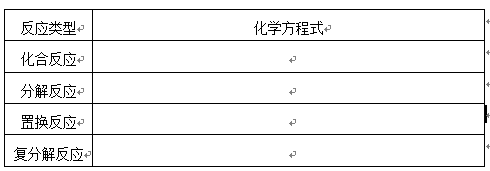
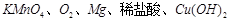 �������ʣ���������Ϊ��Ӧ����±��е�Ҫ���д��һ����ѧ����ʽ��
�������ʣ���������Ϊ��Ӧ����±��е�Ҫ���д��һ����ѧ����ʽ��