��Ŀ����
(7��)�ڻ�ѧ��ȤС���У�һ���С���ʯ�Ҹ��������Сֽ��������ͬѧ�ǵ���Ȥ������ͻ�ѧ��ȤС���ͬѧ��һ��ȥ̽����
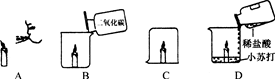
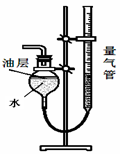
��ʵ��̽��һ��ȡ������Ʒ��С�ձ��У�������ˮ���������ڣ����ȸС���������ʵ��(����ʵ�������������ɢʧ)������Ϊ�˸���� (�û�С�������"��ȫ��")���ʡ� ��
��ʵ��̽��--]ȷ�����ʵĸ�����ɷ֡�
���������롿����һ�����ʵĸ�����ɷ����������ơ�
����������ʵĸ�����ɷ��� ��
�����������ʵĸ�����ɷ���
����֤���롿С����Ƶ�ʵ�鷽����ȡ������Ʒ�����Թ��У���ˮ���ã��²��в�����ϲ���Һ�е����̪��Һ����Һ��죬����ʵĸ�����ɷ����������ơ�ͬѧ�Ǿ����������ۺ�һ����ΪС����ʵ�鷽��������������Ϊ�������������� �������ڲ�����Ͳ�������ѡ����һ�������ʵ�����̽������������ʵ�鱨�棺
��ʵ��̽��һ��ȡ������Ʒ��С�ձ��У�������ˮ���������ڣ����ȸС���������ʵ��(����ʵ�������������ɢʧ)������Ϊ�˸���� (�û�С�������"��ȫ��")���ʡ� ��
��ʵ��̽��--]ȷ�����ʵĸ�����ɷ֡�
���������롿����һ�����ʵĸ�����ɷ����������ơ�
����������ʵĸ�����ɷ��� ��
�����������ʵĸ�����ɷ���
����֤���롿С����Ƶ�ʵ�鷽����ȡ������Ʒ�����Թ��У���ˮ���ã��²��в�����ϲ���Һ�е����̪��Һ����Һ��죬����ʵĸ�����ɷ����������ơ�ͬѧ�Ǿ����������ۺ�һ����ΪС����ʵ�鷽��������������Ϊ�������������� �������ڲ�����Ͳ�������ѡ����һ�������ʵ�����̽������������ʵ�鱨�棺
| ʵ����� | ʵ������ | ʵ����� |
| | | |
(7��)��ʵ��̽��һ��ȫ��
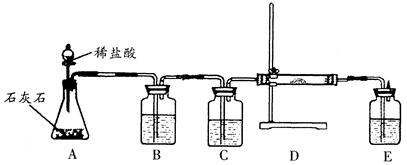
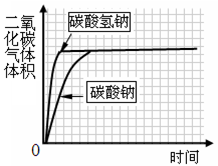
��ʵ��̽��������������������ƺ�̼���
��������̼���
����֤���롿�²�IJ����������̼��ƣ�����˵�����ʵĸ�����ɷ�ֻ����������
�����
(ʵ�����������������ѡ�����Ӧ����������)
��ʵ��̽��������������������ƺ�̼���
��������̼���
����֤���롿�²�IJ����������̼��ƣ�����˵�����ʵĸ�����ɷ�ֻ����������
�����
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��У���ˮ���ã����ϲ���Һ�е����̪��Һ��ȡ�²㲻����μ����� | �²��в�����ϲ���壬��Һ��������ݲ��� | ���ʵĸ�����ɷ�Ϊ�������ƺ�̼��� |
��ʵ��̽��һ��ȡ�����ˮû�зų��ȣ�˵�������в����������ƣ�
�ʴ�Ϊ��ȫ����
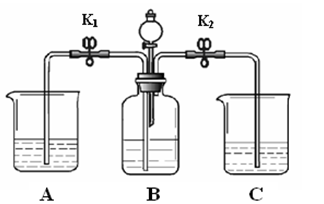
��ʵ��̽��������������������Ʋ������տ����ж�����̼����̼���ʱ����������Ϊ�������ƺ�̼��ƵĻ���
�ʴ�Ϊ���������ƺ�̼��ƣ�
����������������ȫ�����տ����ж�����̼����̼���ʱ����������Ϊ̼��ƣ�
�ʴ�Ϊ��̼��ƣ�
����֤���롿С��û����֤�²�IJ������Ƿ�Ϊ̼��ƣ��͵ó����ۣ�������ý��۲�����˵������
�ʴ�Ϊ���²�IJ����������̼��ƣ�����˵�����ʵĸ�����ɷ�ֻ���������ƣ�
�������������Ϊ�������ƺ�̼��ƵĻ���ȡ��������ˮʱ��������ҺӦ��ʹ��̪��죬���²�����������ϡ�����ܷų����������̼��
�ʴ�Ϊ��
��������������ֻ����̼��ƣ�ȡ��������ˮʱ�������ϲ���Һ����ʹ��̪��죬ֻ�ܹ۲쵽�²������ϡ����ų����������̼��
�ʴ�Ϊ��
��ʵ�����������������ѡ�����Ӧ���������ɣ�
�ʴ�Ϊ��ȫ����
��ʵ��̽��������������������Ʋ������տ����ж�����̼����̼���ʱ����������Ϊ�������ƺ�̼��ƵĻ���
�ʴ�Ϊ���������ƺ�̼��ƣ�
����������������ȫ�����տ����ж�����̼����̼���ʱ����������Ϊ̼��ƣ�
�ʴ�Ϊ��̼��ƣ�
����֤���롿С��û����֤�²�IJ������Ƿ�Ϊ̼��ƣ��͵ó����ۣ�������ý��۲�����˵������
�ʴ�Ϊ���²�IJ����������̼��ƣ�����˵�����ʵĸ�����ɷ�ֻ���������ƣ�
�������������Ϊ�������ƺ�̼��ƵĻ���ȡ��������ˮʱ��������ҺӦ��ʹ��̪��죬���²�����������ϡ�����ܷų����������̼��
�ʴ�Ϊ��
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��У���ˮ���ã����ϲ���Һ�е����̪��Һ��ȡ�²㲻����μ����� | �²��в�����ϲ���壬��Һ��죬�����ݲ��� | ���ʵĸ�����ɷ�Ϊ�������ƺ�̼��� |
�ʴ�Ϊ��
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��У���ˮ���ã����ϲ���Һ�е����̪��Һ��ȡ�²㲻����μ����� | �²��в�����ϲ���壬��Һ����ɫ�������ݲ��� | ���ʵĸ�����ɷ�Ϊ̼��� |

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
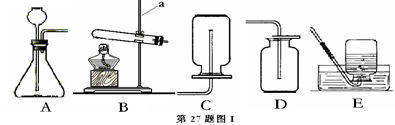
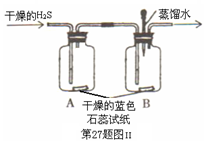

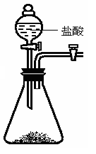

 Cu+H20��CO+CuO
Cu+H20��CO+CuO