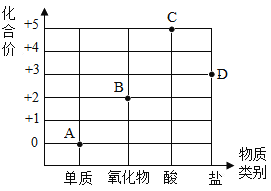
��Ŀ����
����Ŀ��ˮ����Һ������������������ʮ����Ҫ�����ã���ش��������⡣
��1�������е�������ˮ�Ǿ�����ˮ�������������ġ���ʱ������____________ (����������)����ˮ�����ɵĽ�״������ʵ�������ʹ���ʳ������ﵽ��ˮ��Ŀ�ġ�
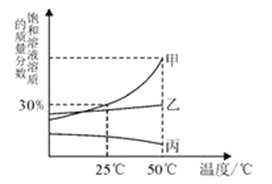
��2��ˮ�dz������ܼ���A��B��C���ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ��ʾ��
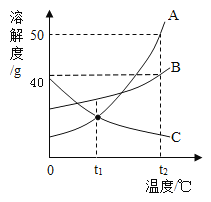
��t2��ʱ��A �ı�����Һ�����ʵ���������Ϊ ____________ (����һλС��)
������˵����ȷ������__________��
A. t2��ʱ��A��B��C �������ʵ��ܽ�ȴ�СΪ��A��B��C
B.�� t2��ʱ A��B��C �ı�����Һ�քe������ t1�棬������Һ�����ʵ�����������СΪ��B��A=C
C.�Ӻ������� B�� A�ı�����Һ�еõ��϶�� A ���壬ͨ���ɲ�����ȴ�ȱ�����Һ�ķ���
��3��ѹǿΪ 101 kPa ���¶�Ϊ 0��ʱ��������ˮ�е��ܽ��Ϊ 0.024����仰�ĺ�����______ ��
��4����ͼʵ�߷������ǻ�ѧ��Ӧ�����ͣ��������߷������Ƕ�Ӧ��һ����ˮ�μӵķ�Ӧ�� ��ѧ����ʽ������ͼ����ա�
���Ϸ�Ӧ | H2O+CO2=H2CO3 |
�ֽⷴӦ | ____________ |
�û���Ӧ | Fe+4H2O |
���� | 6H2O+6CO2 |
��5�����л�ѧ�У�����ѧϰ��ϡ���������������Һ����ͼ��ʾ�������кͷ�Ӧ��ʵ���ǣ�H++OH= H2O����������ʵ�ʲμ� ��Ӧ�����ӷ�������ʾ��Ӧ��ʽ�ӽ����ӷ���ʽ����ϡ��������� ������Һ��ӦΪ�������ӷ���ʽ����дһ�㰴���²��裺
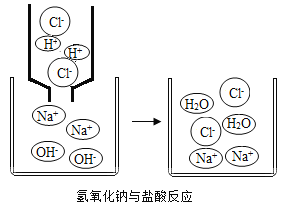
��.д��ϡ����������������Һ��Ӧ�Ļ�ѧ����ʽ____________��
��.��������ˮ����������д��������ʽ�������ܵ����ʡ������ˮ�����û�ѧʽ��ʾ�� ��������ʽ�ɸ�д�ɣ�H++Cl++Na++OH- = Na++Cl-+H2O��
��.ɾȥ����ʽ���߲��μӷ�Ӧ�����ӣ�������д�ɣ�H++OH=H2O��
��.��鷽��ʽ���߸�Ԫ�ص�ԭ�Ӹ����͵�������Ƿ���ȡ���ϡ���������������Һ��Ӧ�����ӷ���ʽΪ: H++OH- = H2O����ش�
��6��д��ϡ�����̼��Ʒ�����Ӧ�����ӷ���ʽ____________��
��7���ᡢ�����ˮ��Һ�з����ĸ��ֽⷴӦʵ���Ͼ������ֻ���������Һ��������� �ӵķ�Ӧ������������____________����ʱ�����ֽⷴӦ�ſ��Է�����
���𰸡����� 33.3% AC 101kPa��0��ʱ��1���ˮ������ܽ� 0.024 ����ĵ��� 2H2O![]() 2H2��+O2�� Fe3O4�� NaOH+HCl=NaCl+H2O�� CaCO3+2H+=Ca2++H2O+CO2���� ˮ����������
2H2��+O2�� Fe3O4�� NaOH+HCl=NaCl+H2O�� CaCO3+2H+=Ca2++H2O+CO2���� ˮ����������
��������
��1������ˮ�Ĵ��������У�ʹ����������ˮ�����ɽ�״������ʵ��������ʴ�Ϊ��������
��2���ٸ��������֪��t2��ʱ��A �ı�����Һ�����ʵ���������=![]() ���ʴ�Ϊ��33.3%��
���ʴ�Ϊ��33.3%��
��A.t2��ʱ��A��B��C �������ʵ��ܽ�ȴ�СΪA��B��C��A��ȷ��
B.��t2��ʱ A��B��C �ı�����Һ�քe������t1�棬A��B�о���������C����������������Һ�����ʵ�����������СΪB��A��C��B����
C.�Ӻ������� B�� A�ı�����Һ�еõ��϶�� A ���壬ͨ���ɲ�����ȴ�ȱ�����Һ�ķ�����C��ȷ���ʴ�ΪAC��
��3��ѹǿΪ 101 kPa ���¶�Ϊ 0��ʱ��������ˮ�е��ܽ��Ϊ 0.024����仰�ĺ�����101kPa��0��ʱ��1���ˮ������ܽ� 0.024 ����ĵ�����
��4���ֽⷴӦ�����ǣ�2H2O![]() 2H2��+O2������ΪFe+4H2O
2H2��+O2������ΪFe+4H2O![]() Fe3O4+4H2���ʴ�Ϊ��Fe3O4
Fe3O4+4H2���ʴ�Ϊ��Fe3O4
��5��ϡ����������������Һ��Ӧ�Ļ�ѧ����ʽ��NaOH+HCl=NaCl+H2O
��6��̼��Ʋ�����ˮ�����ܲ�ϡ�����̼��Ʒ�����Ӧ�����ӷ���ʽΪ��CaCO3+2H+=Ca2++H2O+CO2��
��7�����ֽⷴӦ��������������ˮ������������һ�����ɣ��ʴ�Ϊ��ˮ����������

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�����Ŀ����һ�����Ĺ㷺Ӧ��
��1�����������͡��е���ָ����______ ������ĸ����
A ����
B Ԫ��
C ԭ��
��2�����ƴ�����ʳ�˵�������е�������_____ ��
��������������������ɵIJⶨ
�����������壨��ѧ���ΪFeC2O4��xH2O����һ�ֵ���ɫ�����ĩ��
���������ϣ���1��CO�����Ȼ��٣�PdCl2����Һ��Ӧ���ɺ�ɫ���ٷۣ�
��2��FeO��Fe3O4���Ǻ�ɫ���ʣ�FeO�ڿ����в��ȶ����ױ����������������������ȷֽ��������������Ϊȷ���ֽ���Px��ֵ���������ʵ�顣
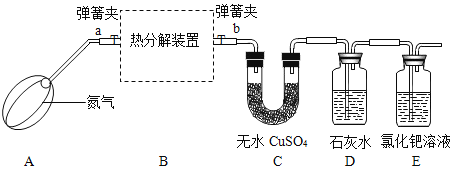
��ʵ����ƣ���װ��C��D��E��ҩƷ��������
��С��ʿ����ɫ����ˮCuSO4����ˮ�ֺ����ɫ
��ʵ�鲽�裩
��1������װ�ã����________��
��2����װ��C��������
��3����װ��B��װ��9.0g�IJ����������壬���ɼ�a��b������һ����������
��4���þƾ��Ƽ���Bװ�ã�ֱ��������ȫ��ɺ�ɫ��ֹͣ���ȣ�
��5������ͨ������װ����ȴ���رյ��ɼ�a��b��
��6���ٴγ���װ��B�й���������װ��C��������
������ʵ�飩��С��ͬѧ������ʵ�鲽�����ʵ�飬����¼���������ݣ�
װ��B�й���������/g�� | װ��C��������/g�� | |
ʵ��ǰ | 9.0 | 168.2 |
ʵ��� | 3.6 | 170.0 |
��ʵ����������ݴ�����
��1������ǰ�ȹ���һ��ʱ�䵪����Ŀ����_______��
��2��ʵ������У�װ��C�й����Ϊ��ɫ��˵��������������ֽ�������_____FeC2O4��xH2O��x��ֵΪ___��ʵ���������������ͨ������Xֵ��____���ƫ�������䡱��ƫС������
��3��װ��D��ʯ��ˮ����ǣ�˵��������������ֽ������_____��
��4��װ��E���ֺ�ɫ���ʣ�˵��������������ֽ������_____��ͬʱװ��E�����е�����������___����ֹ��Ⱦ������
��5�������ϱ����ݣ����㷴Ӧ��װ��B�в�����ɫ���ʵĻ�ѧʽ_____��
��6��ʵ���װ��B�й������ɫ�ɵ���ɫ��ɺ�ɫ��д�����������������ȷֽ�Ļ�ѧ����ʽ______��