��Ŀ����
��ľ̿��ԭ����ͭ��ʵ����ͼ��
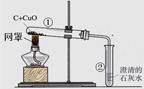
(1)�ƾ��Ƽӵ��ֵ�Ŀ�� ��
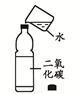
(2)�տ�ʼԤ�ȣ��Թܢ��������������ݣ���ʯ��ˮ������ǣ�ԭ���� ��
(3)�������ȣ��۲쵽ʯ��ˮ����ǣ���ɫ��ĩ�г��ֺ�ɫ���ʡ�����д�����㲿�ֵĻ�ѧ����ʽ�� �� ��
(4)ֹͣ����ʱ��Ӧ�Ƚ����ܴ��Թܢ��г��������õ��ɼмн���Ƥ�ܣ����Թܢ���ȴ���ٰ��Թ���ķ�ĩ����������������ԭ���� ��

(1)�ƾ��Ƽӵ��ֵ�Ŀ�� ��
(2)�տ�ʼԤ�ȣ��Թܢ��������������ݣ���ʯ��ˮ������ǣ�ԭ���� ��
(3)�������ȣ��۲쵽ʯ��ˮ����ǣ���ɫ��ĩ�г��ֺ�ɫ���ʡ�����д�����㲿�ֵĻ�ѧ����ʽ�� �� ��
(4)ֹͣ����ʱ��Ӧ�Ƚ����ܴ��Թܢ��г��������õ��ɼмн���Ƥ�ܣ����Թܢ���ȴ���ٰ��Թ���ķ�ĩ����������������ԭ���� ��
��1��ʹ���漯�еIJ�����¶ȣ�2����ʼ�ų������Թ��ڵĿ���
��3��C+2CuO 2Cu+CO2������CO2+Ca(OH)2==CaCO3 ��+H2O
2Cu+CO2������CO2+Ca(OH)2==CaCO3 ��+H2O
(4)��ֹ����Ŀ������벣����ʹͭ���±�����
��3��C+2CuO
 2Cu+CO2������CO2+Ca(OH)2==CaCO3 ��+H2O
2Cu+CO2������CO2+Ca(OH)2==CaCO3 ��+H2O(4)��ֹ����Ŀ������벣����ʹͭ���±�����
�����������1�����ݾƾ��Ƶ�ʹ�ý��з������ƾ��Ƽӵ��ֿ��Լ��л��棬����¶ȣ�
��2�������Թ����п���������ʱ����������ͣ����ȳ����������ǿ����������еĶ�����̼���٣���ʯ��ˮ������ǣ�
��3��ľ̼�ڸ����»�ԭ����ͭ���к�ɫ��ͭ�Ͷ�����̼���ɣ���Ӧ�Ļ�ѧ����ʽΪC+2CuO
 2Cu+CO2�������ɵĶ�����̼�����ʯ��ˮ��Ӧ������̼��Ƴ�����ˮ������ʹ����ʯ��ˮ����ǣ���Ӧ�Ļ�ѧ����ʽΪCO2+Ca(OH)2=CaCO3 ��+H2O��
2Cu+CO2�������ɵĶ�����̼�����ʯ��ˮ��Ӧ������̼��Ƴ�����ˮ������ʹ����ʯ��ˮ����ǣ���Ӧ�Ļ�ѧ����ʽΪCO2+Ca(OH)2=CaCO3 ��+H2O����4��ֹͣ����ʱ���Ƚ����ܴ��Թܢ��г������ɷ�ֹ�Թܢ��е�ʯ��ˮ�������ȵ��Թܣ�ʹ�Թ�ը�ѣ� �õ��ɼмн���Ƥ�ܣ����Թܢ���ȴ���ٰ��Թ���ķ�ĩ�������ɷ�ֹ���������Թܢ٣���ʹ�Թܢ������ɵ����ȵ�ͭ�ٴα�������
������������Ҫ������̼��ԭ����ͭ��ʵ�������ʵ����������������Ŀ����Ҫ��Ǻ�����̼��ԭ����ͭʵ��Ļ�ѧ��Ӧ����ʽ��������ע������������Լ�������̼�ļ�������֪ʶ��ֻ���������ܶ���ط��������������ȷ�Ľ��

��ϰ��ϵ�д�
�����Ŀ