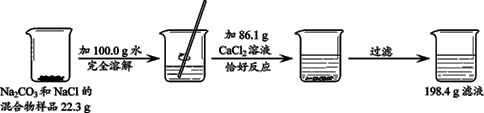��Ŀ����
(5��)ij��ѧ��ȤС�����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ20gʯ��ʯ��Ʒ(�������ʼȲ�����ˮ��Ҳ�����������ʷ�Ӧ)�������м���100g������������Ϊ10��95����ϡ���ᣬǡ����ȫ��Ӧ����ش��������⡣
(1)������100g����ʵ�����õ�ϡ���ᣬ��Ҫ36��5����Ũ��������Ϊ________________
(2)д����Ӧ�Ļ�ѧ����ʽ ________________
(3)�г�������Ʒ��μӷ�Ӧ��̼�������(x)�ı���ʽ ________
(4) ����Ʒ��̼��Ƶ���������Ϊ________
(5)��Ӧ��Ĺ�Һ������м���l13��6gˮ����ֽ������ˣ��õ�ֻ��һ�����ʵIJ�������Һ�������ò�������Һ�����ʵ���������Ϊ________________
(1)������100g����ʵ�����õ�ϡ���ᣬ��Ҫ36��5����Ũ��������Ϊ________________
(2)д����Ӧ�Ļ�ѧ����ʽ ________________
(3)�г�������Ʒ��μӷ�Ӧ��̼�������(x)�ı���ʽ ________
(4) ����Ʒ��̼��Ƶ���������Ϊ________
(5)��Ӧ��Ĺ�Һ������м���l13��6gˮ����ֽ������ˣ��õ�ֻ��һ�����ʵIJ�������Һ�������ò�������Һ�����ʵ���������Ϊ________________
��1��30g
��2��CaCO+2HCl=CaCl2+H2O+CO2����3��100/73="X/10.95g" (4)7.5%
��2��CaCO+2HCl=CaCl2+H2O+CO2����3��100/73="X/10.95g" (4)7.5%
��������1��������������һ����������������������ʽ���м��㼴�ɣ�
��2��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ݴ�д����ѧ����ʽ���ɣ�
��3��������֪����������Һ�����ʵ���������Ȼ�����̼��������ᷴӦ�Ļ�ѧ����ʽ�����ɵó�������Ʒ�вμӷ�Ӧ��̼���������x���ı���ʽ��
��4���ɣ�3���м�����ĸ���Ʒ�вμӷ�Ӧ��̼�����������������������ʽ�� ��Ʒ�вμӷ�Ӧ��̼�������/��Ʒ��������100%�����㼴�ɣ�
��5�����ò�������Һ������=��Ӧǰ���ʵ�������-��������-������̼������+����ˮ�������������������ǣ�3���м���������ʣ�CaCl2����������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��1��������������ϡ��ǰ�䣻����Ҫ36.5%��Ũ��������Ϊa
100g��10.95%=a��36.5%
a=30g
��2��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3�������Ʒ�вμӷ�Ӧ��̼�������Ϊx�������Ȼ��Ƶ�����Ϊy�����ɶ�����̼������Ϊz��
CaCO3 +2HCl�TCaCl2 +H2O+CO2��
100 73 111 44
x 100g��10.95% y z
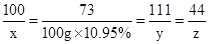
��֮�ã�x=15g��y=16.65g��z=6.6g��
��4������Ʒ��̼��Ƶ���������=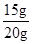 ��100%=75%��
��100%=75%��
��5��ʯ��ʯ��Ʒ�����ʵ�����Ϊ��20g-15g=5g��
���ò�������Һ������Ϊ��100g+20g-5g-6.6g+113.6g=222g��
�����ò�������Һ�����ʵ���������Ϊ��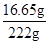 ��100%=7.5%��
��100%=7.5%��
�ʴ�Ϊ����1��30g
��2��CaCO3 +2HCl�TCaCl2 +H2O+CO2��
��3��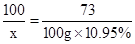
��4��75%
��5��7.5%
��2��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ݴ�д����ѧ����ʽ���ɣ�
��3��������֪����������Һ�����ʵ���������Ȼ�����̼��������ᷴӦ�Ļ�ѧ����ʽ�����ɵó�������Ʒ�вμӷ�Ӧ��̼���������x���ı���ʽ��
��4���ɣ�3���м�����ĸ���Ʒ�вμӷ�Ӧ��̼�����������������������ʽ�� ��Ʒ�вμӷ�Ӧ��̼�������/��Ʒ��������100%�����㼴�ɣ�
��5�����ò�������Һ������=��Ӧǰ���ʵ�������-��������-������̼������+����ˮ�������������������ǣ�3���м���������ʣ�CaCl2����������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��1��������������ϡ��ǰ�䣻����Ҫ36.5%��Ũ��������Ϊa
100g��10.95%=a��36.5%
a=30g
��2��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3�������Ʒ�вμӷ�Ӧ��̼�������Ϊx�������Ȼ��Ƶ�����Ϊy�����ɶ�����̼������Ϊz��
CaCO3 +2HCl�TCaCl2 +H2O+CO2��
100 73 111 44
x 100g��10.95% y z
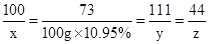
��֮�ã�x=15g��y=16.65g��z=6.6g��
��4������Ʒ��̼��Ƶ���������=
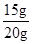 ��100%=75%��
��100%=75%����5��ʯ��ʯ��Ʒ�����ʵ�����Ϊ��20g-15g=5g��
���ò�������Һ������Ϊ��100g+20g-5g-6.6g+113.6g=222g��
�����ò�������Һ�����ʵ���������Ϊ��
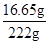 ��100%=7.5%��
��100%=7.5%���ʴ�Ϊ����1��30g
��2��CaCO3 +2HCl�TCaCl2 +H2O+CO2��
��3��
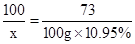
��4��75%
��5��7.5%

��ϰ��ϵ�д�
�����Ŀ