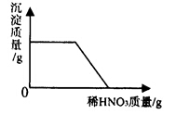
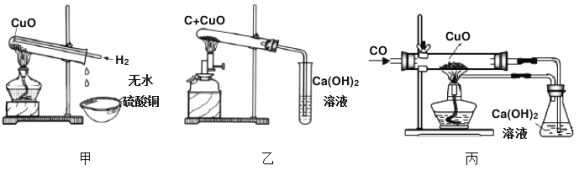
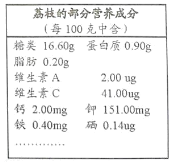
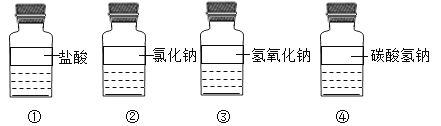
��Ŀ����
����Ŀ����ѧ�����������ܲ��ɷ֡�
��1������ͨ��ʳ���ȡ����Ӫ����
��ˮ�����߲˸�����Ӫ������Ҫ��ˮ�� ��
������ʳ���У����ṩ���������ʵ��� ������ĸ��ţ���

��2�����г�����Ʒ��ʹ�õ���Ҫ���ϣ������л��ϳɲ��ϵ��� ������ĸ��ţ���

��3�������Ի�ʯȼ��Ϊ��Ҫ��Դ��
����Ȼ��ȼ�յĻ�ѧ����ʽΪ ��
���������������ڼ��ٿ�����Ⱦ���� ������ĸ��ţ���
A���������̫���� B��������չ��������
C���Ӵ������Դ�ı��� D��Ϊ������У�����ʹ��˽�ҳ�
���𰸡���1����ά���� �� C ��2��C��D
��3����CH4+2O2![]() 2H2O + CO2 �� AC
2H2O + CO2 �� AC
��������
�����������1����ˮ�����߲˸�����Ӫ������Ҫ��ˮ��ά����
�����A�⡢Ƥ����ë�����㡢�ǵ���Ҫ�ɷ֡����ࡢ���ࡢ���ࡢţ�⡢�����ж����зḻ�ĵ����ʣ���ѡC
��2���л��ϳɲ��ϰ��������ϡ��ϳ���ά���ϳ���A���մ����������ǽ������ϣ�B����������ڽ������ϣ�C��D ���ںϳɲ���
��3������Ȼ������Ҫ�ɷ��Ǽ��飬ȼ�յĻ�ѧ����ʽΪCH4+2O2��ȼ2H2O + CO2
�� A���������̫���������ڼ��ٿ�����Ⱦ����ȷ��B������������Ҫ������úȼ�շ��ȣ���ȼ��ú�Կ�������Ⱦ������C���Ӵ������Դ�ı��������ڼ��ٿ�����Ⱦ����ȷ��D������ʹ��˽�ҳ���������β�����ŷŶԿ�������Ⱦ������ѡAC

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�