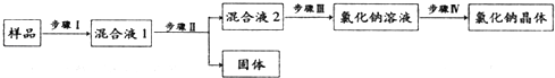
��Ŀ����
����Ŀ����12�֣�С���ڻ�ѧʵ�鼼�ܿ����У���ɡ���ϡ�����������������Һ��̼������Һ������̼������Һ��pH��ʵ�顣
��ʵ��һ��С��ȡ����Һ�ס��ҷֱ�������Թ���������������֧�Թ��е���ϡ���ᣬ�۲�ʵ��������������֪����Ϊ̼������Һ��
��1��ʵ���У�С��Ӧ�۲쵽�Թ�����������__________________________________��
���Թ��з�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��2����ʦָ�����������⣬����ʹ�������Լ������������ƺ�̼������Һ������ѡ��һ�������������Լ�����������ʵ���е�Ԥ�������Լ���ѧ����ʽ�����±���
ѡ����Լ� | Ԥ������ | ��Ӧ�Ļ�ѧ����ʽ |
____________ | һ֧�Թ����������� ��һ֧�Թ�________________ | _________________________ |
��ʵ�����С���ò�����պȡ��ʵ��һ�����Թ����е���Һմ��pH��ֽ�ϣ��۲���ֽ��ɫ�仯�������ɫ�����գ�������Һ��pH��С��ļ�¼���£�
ʵ������ | ���� |
��Һմ����ֽ��ʱ����ֽ�����ر�ɫ | �����ɫ���Աȣ�Na2CO3��ҺpH=3 |
��3��С����������ó���Na2CO3��ҺpH=3���Ĵ�����ۡ�ָ��С���������֮����
��_______________________________________________________________��
��4��ָ��С���¼�ġ�ʵ�������в���ѧ�ĵط���
��_______________________________________________��
���𰸡� ������������ð��������Na2CO3+2HCl=2NaCl+H2O+CO2��(2)
ѡ����Լ� | Ԥ������ | ��Ӧ�Ļ�ѧ����ʽ |
Ca(OH)2��Һ | ������ɫ���������ְ�ɫ���ǣ� | Ca(OH)2+Na2CO3=CaCO3��+2NaOH |
������С����ȡ��ҺΪ������̼���Ʒ�Ӧ�����Һ��������ֽ������ɫ���ǻ����ر�ɫ
��������
�����������ʵ��һ��С��ȡ����Һ�ס��ҷֱ�������Թ���������������֧�Թ��е���ϡ���ᣬ�۲�ʵ��������������֪����Ϊ̼������Һ��1��ʵ���У�С��Ӧ�۲쵽�Թ�����������������ð�������䷴Ӧ�ķ���ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2������2����ʦָ�����������⣬����ʹ�������Լ������������ƺ�̼������Һ������ѡ��һ�������������Լ�������Ҫ����дʵ�鷽��Ϊ
ѡ����Լ� | Ԥ������ | ��Ӧ�Ļ�ѧ����ʽ |
Ca(OH)2��Һ | ������ɫ���������ְ�ɫ���ǣ� | Ca(OH)2+Na2CO3=CaCO3��+2NaOH |
��ʵ�������3��С����������ó���Na2CO3��ҺpH=3���Ĵ�����ۡ�ָ��С���������֮����С����ȡ��ҺΪ������̼���Ʒ�Ӧ�����Һ����4��ָ��С���¼�ġ�ʵ�������в���ѧ�ĵط���ֽ������ɫ���ǻ����ر�ɫ��