��Ŀ����
����Ŀ��ijУ��ѧ��ȤС����д���(������ɳ)�ᴿʵ�飬���������þ�������100g20�����Ȼ�����Һ��
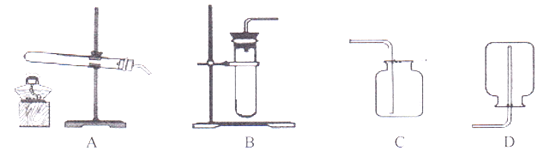
ʵ��һ��ͼ1�Ǽ�ͬѧ���д����ᴿʵ��IJ���ʾ��ͼ
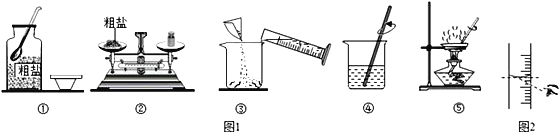
(1)ͼ���е�һ�����Դ�����______________
(2)��ͬѧʵ����ȱ�ٹ��˲������ڴ˲����в������������ǣ�______________
ʵ�������ͬѧ���ᴿ�õ��ľ�������100g20�����Ȼ�����Һ
(3)����ʱ����ͼ1�ṩ�������⣬����Ҫ�õ��Լ�ƿ�����Ӻ�______________(��һ������������)
(4)��������������ȷ����ȡͼ2�ķ�ʽ��ȡˮ����������Һ��������������______________(����ڡ�С�ڻ����)20��
(5)�������У�ֹͣ���ȵ�ǡ��ʱ��ʱ����������������______________
���𰸡� ƿ��û�е��� ���� ��ͷ�ι� С�� ���ֽ϶����ʱ ��ֹ�ֲ��¶ȹ��ߣ����Һ��ɽ�
����������1��ȡ��ҩƷʱ��ƿ��Ҫ���ţ���2������ʱҪ�ò�������������3��������Һ��һ�㲽���ǣ����㡢�������ܽ⣬�ʻ���Ҫ��ͷ�ιܣ���4����ȡͼ2�ķ�ʽ��ȡˮ���ᵼ��������ˮ�����ƫ����Һ������ƫ����ô�����Ƶ���Һ����������������ƫС����5������ʱҪ���ȱ߽��裬Ŀ���Ƿ�ֹ�ֲ��¶ȹ��ߣ����Һ��ɽ��������ֽ϶����ʱֹͣ���ȣ��������Ƚ�Һ�����ɡ�

����Ŀ����д��ѧ���Ż�ѧ���ű�ʾ������
��ѧ���� | ____ | _______ |
| NO3- | _______ | ||
��ʾ���� | ���� | ���������� | _______ | _______ | 2���������� |
����Ŀ��ij�о�С��Ϊ̽���������ڲ�ͬ��Һ�и�ʴ�����ʡ�������ͬ������ȡͬŨ�ȵ����в�ͬ��Һ����ͼװ�����ʵ�飬�����ֳ����ݲɼ������вⶨ���ش��������⣺
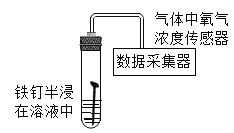
������Һ | NH4Cl | (NH4)2SO4 | NH4NO3 |
0minʱ����Ũ��(%) | 20.8 | 20.8 | 20.8 |
500minʱ����Ũ��(%) | 15.8 | 17.0 | 17.4 |
ʵ��ǰ��ҺPH | 5.31 | 5.53 | 5.50 |
ʵ�����ҺPH | 7.37 | 7.99 | 8.22 |
����������ʴ��� | �������� | �������� | �������� |
��1��������Ҫ�ɷֵĻ�ѧʽΪ____________
��2����NH4Cl��(NH4)2SO4����NH4NO3��Һ�У�������ʴ������������Һ�����Ծ���____���ǿ����������
��3������ʵ�鷽���Ƿ������________________������������������������������______
��4��������ʴ�����ʻ�������________________�йء���������ʵ�飬��֤��IJ��룺
ʵ����� | ʵ�������� |
__________________ | _______________________ |
��5����С���ڲ�ͬ�������Һ���ֽ�����̽��������ͬ��������ⶨ��ʵ����������ͼ��
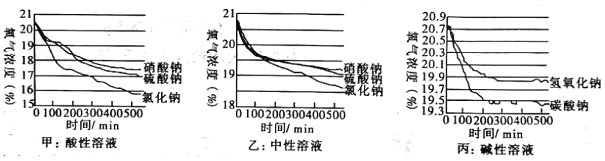
a������ʵ���У�����Ũ���½��ȶ���ԭ��Ϊ____________________
b�����ݼס���ͼ����д��������ۣ�
�ٽ��ۣ�____________________________
�ڽ��ۣ�___________________________
�۽��ۣ�____________________________