��Ŀ����
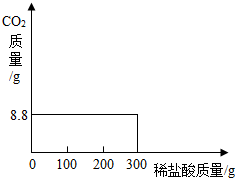
���ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õļ�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3������25.0g���������Ƴ���Һ������������μ���������������������Ϊ7.3%��ϡ���ᣬʹ������ȫ�ų������ռ���8.8g������̼���壮�Լ��㣺��1��ԭ������Na2CO3��������������
��2����Ӧ�����ĵ��������������
��������1���ɸ��ݶ�����̼���������̼���Ƶ��������ٸ���
��100%���ԭ������̼���Ƶ�����������
��2�����ݶ�����̼���������������Һ�����ʵ��������ٸ���
����������������
| ̼���Ƶ����� |
| ��������� |
��2�����ݶ�����̼���������������Һ�����ʵ��������ٸ���
| ���ʵ����� |
| ������Һ�����ʵ��������� |
����⣺��1����̼���Ƶ�����Ϊx��������Һ�����ʵ�����Ϊy��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 73 44
x y 8.8g
=
x=21.2g
=
y=14.6g
��100%=84.8%
��ԭ������̼���Ƶ���������Ϊ84.8%��
��2����Ӧ�����ĵ������������Ϊ��
=200g �𣺷�Ӧ�����������������Ϊ200g��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 73 44
x y 8.8g
| 106 |
| 44 |
| x |
| 8.8g |
x=21.2g
| 73 |
| 44 |
| y |
| 8.8g |
y=14.6g
| 21.2g |
| 25g |
��ԭ������̼���Ƶ���������Ϊ84.8%��
��2����Ӧ�����ĵ������������Ϊ��
| 14.6g |
| 7.3% |
����������Ǽ�����Σ���̼���ƣ�ֻ����̼���Ƶ�ˮ��Һ�Լ��ԣ�

��ϰ��ϵ�д�
�����Ŀ
 27����Һ�������Ϳ����о��й㷺����;����ش��������⣺
27����Һ�������Ϳ����о��й㷺����;����ش��������⣺
 ��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��
��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��